Fig. 4.
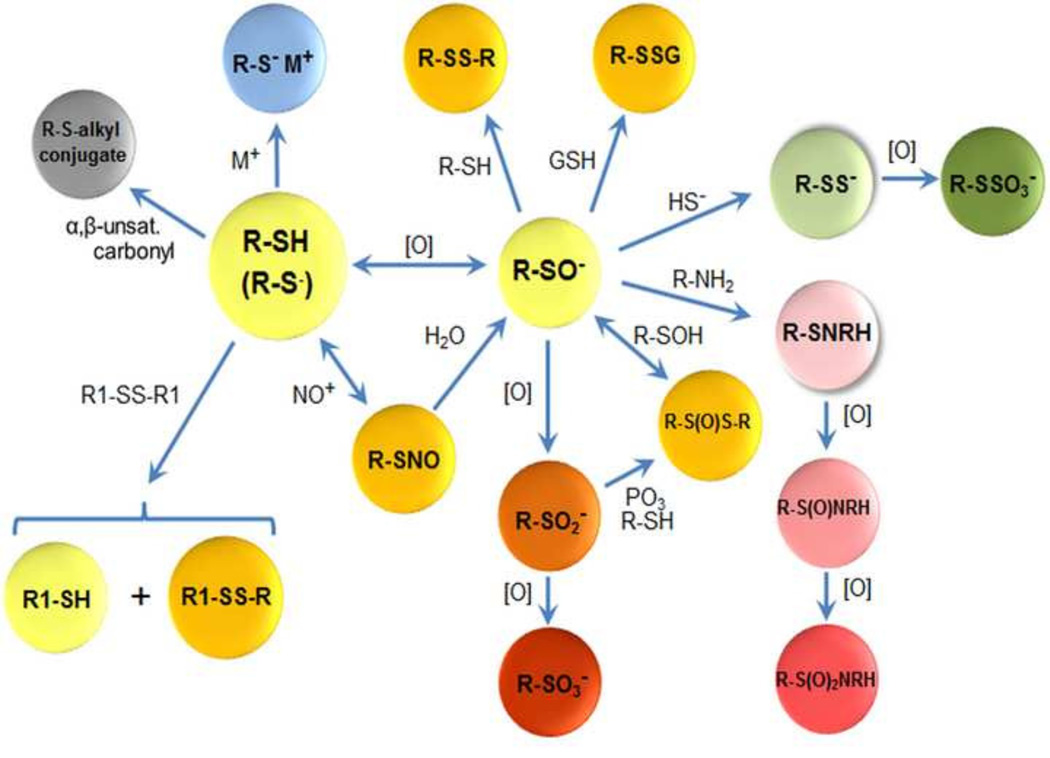
Functional modifications of the Cys proteome. Protein Cys (RSH) undergoes a range of enzymatic and non-enzymatic modifications, often mediated by pH-dependent ionization to Cys thiolate (RS−; large yellow sphere). Interaction of RS− with metal ions (blue) is common as a structural element, as a binding motif for nucleic acids and as a component of catalytic sites. RS− is subject to oxidation ([O]) to Cys sulfenate (RSO−, yellow) by H2O2 and other 2-electron oxidants. Thiol (RSH) also undergoes exchange with disulfide (R1S-SR1) to generate different thiol (R1SH) and disulfide (RS-SR1) in a process termed thiol-disulfide exchange. Non-equilibrium steady-state oxidation of specific RSH occurs due to the presence of low nanomolar concentrations of H2O2 in cells. RSO− reacts with RSH or GSH to produce the respective disulfides (RSSR, RSSG; dark yellow, top). Many RSO− can undergo hyperoxidation to sulfinate (RSO2−; orange, bottom) and sulfonate (RSO3−; red) in the presence of excess oxidant. RS− also reacts with hypothiocyanous acid (HOSCN) to produce sulfenyl thiocyanate (RSSCN; dark yellow, upper left), which rapidly hydrolyzes, and with nitric oxide through multiple means of nitronium ion transfer (NO+) to produce a corresponding nitrosothiol (RSNO; orange, bottom left). RSO− can also react with hydrogen sulfide (HS−) to form a cysteinyl persulfide (RSS−, green), which can be oxidized to a cysteinyl thiosulfate (RSSO3−). Alternatively, RSO− can react with primary amines of neighboring amino acids or separate biomolecules to form sulfenamide (RSNHR) which can be further oxidized to sulfinamide (RS(O)NHR) and sulfonamide (RS(O)2NHR), frequently resulting in cyclic intramolecular structures.