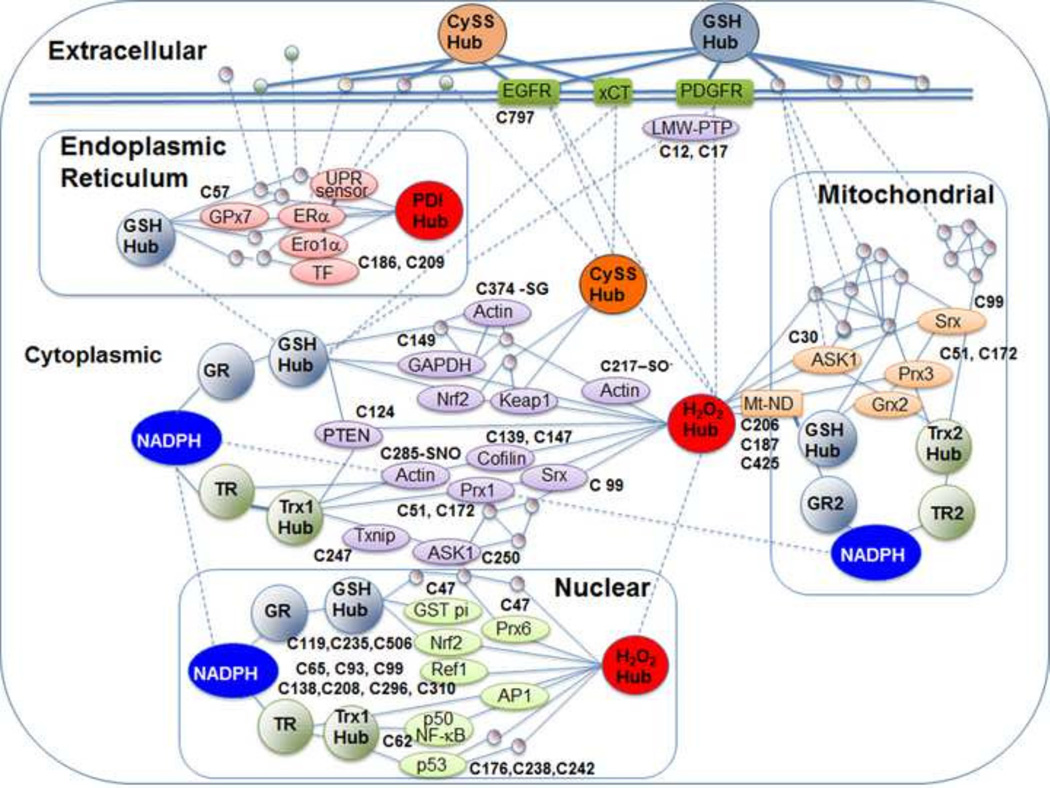
Fig. 5.
Cys proteome networks regulated by Trx and GSH systems. Accumulating evidence shows that redox networks exist in different subcellular compartments supported by NADPH as reductant functioning in opposition to oxidation by H2O2 and other oxidants. Trx is the most common intermediary reductant, with a smaller number of proteins supported by GSH. Cysteine and cystine constitute the most abundant extracellular low molecular weight thiol/disulfide couple, apparently functioning in regulation of protein Cys on the extracellular surface. References provide detailed information on interactions of Cys proteins and functional Cys residues illustrated in this figure. TrxR-actin [110]; Trx-dependent regulation of proteins [139]; Ref1-AP1 [258]; TrxR/AP1 [259]; PDGFR [260]; xCT [261]; PTEN-Trx [262, 263]; PTEN-GSH [264]; ASK1-Trx2 [264]; GSTp1-Prx6 [265]; GAPDH-GSH [266, 267]; ND-GSH [268]; p53-Trx [269]; Trx2-Prx3 [270]; Srx-Prx1 and Srx-Prx3 [271, 272]; Grx2-Prx3 [273]; Grx2-Prx3-Trx2 [274]; PDI-UPR sensor [275]; Era-PDI [276]; PDI-TF [277]; Ero-α-PDI and GSH [278]; GPx7-ER [279]; Nrf2 regulatory Cys residues [280]; Srx functional Cys residues [281].