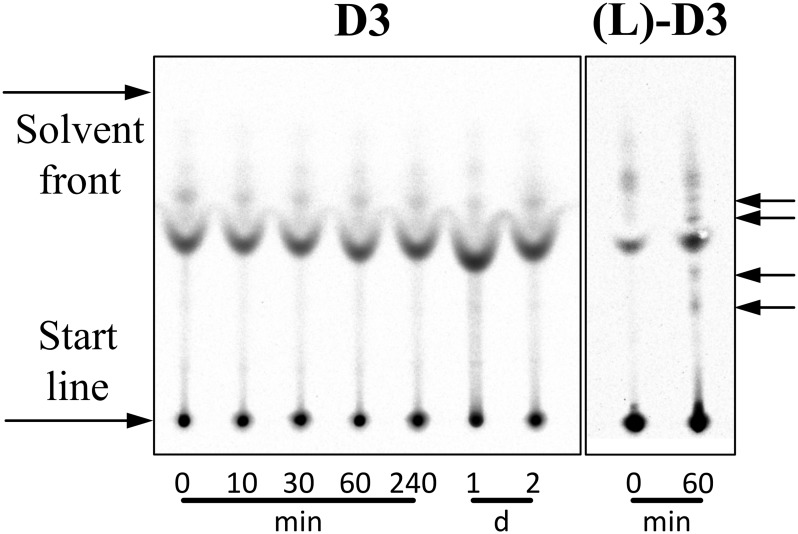
Fig 1. Autoradiogram demonstrating proteolytic stability of 3H labelled peptides in plasma.
3H-D3 was incubated with plasma for different times at 37°C and developed on TLC plates. For comparison, the exact enantiomer of D3, (L)-D3, was used in this stability assay. 3H-(L)-D3 was incubated with plasma for 0 and 60 min at 37°C. Please note that free 3H-(L)-D3 and free 3H-D3 are perfect enantiomers to each other and because the TLC material is not chiral, both compounds show identical Rf values. Additional bands in the 0 min lanes of 3H-(L)-D3 and 3H-D3 that arise from binding and co-migration of 3H-D3 and 3H-(L)-D3 to plasma components do not necessarily have identical Rf values in the 0 min lanes of 3H-(L)-D3 and 3H-D3, because some of the plasma components are enantiomers themselves. Therefore, any effect of degradation will lead to extra additional bands as compared to the 0 min lane of the very same compound. Obvious proteolytic degradation can be observed for 3H-(L)-D3 already after 60 min incubation with plasma leading to additionally appearing bands (black arrows) as compared to the 0 min lane 3H-(L)-D3. Additionally appearing bands as compared to 0 min incubation are not observed for 3H-D3 even after 2 days incubation.