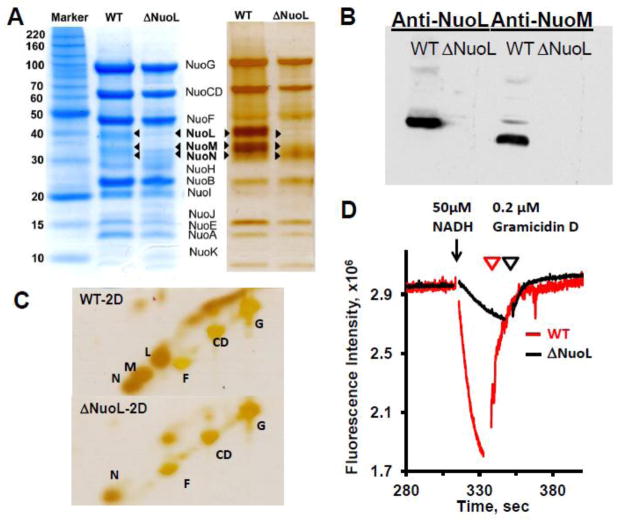
Fig. 1.
SDS-PAGE analyses of purified complex I from the wild-type and the ΔNuoL variant, and their proton translocation activities after reconstitution into proteoliposomes. (A) 1D Tricine SDS-PAGE stained with Coomassie Brilliant Blue (left) and silver (right). (B) Immunoblotting with anti-NuoL and anti-NuoM antibodies. (C) Two dimensional SDS analysis. (D) Generation of a proton gradient monitored by the quench of the ACMA fluorescence. The NADH-DQ and NADH:ferricyanide activities were 12.23 and 58.58, 5.15, and 137.63 μmol/min/mg for the WT and ΔNuoL preparations, respectively. The data were normalized based on the complex I concentrations of the wild-type (MW 537 kDa) and the ΔNuoL variant (MW 417 kDa, lacking NuoL and NuoM) and the complex I orientation factor in the proteoliposomes (79% for the wild-type; 62% for the ΔNuoL variant).