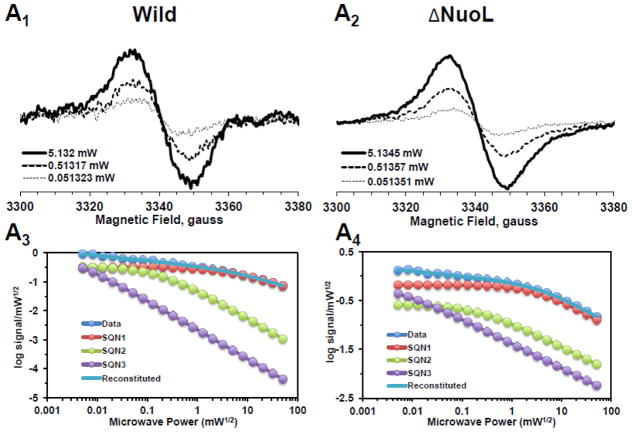
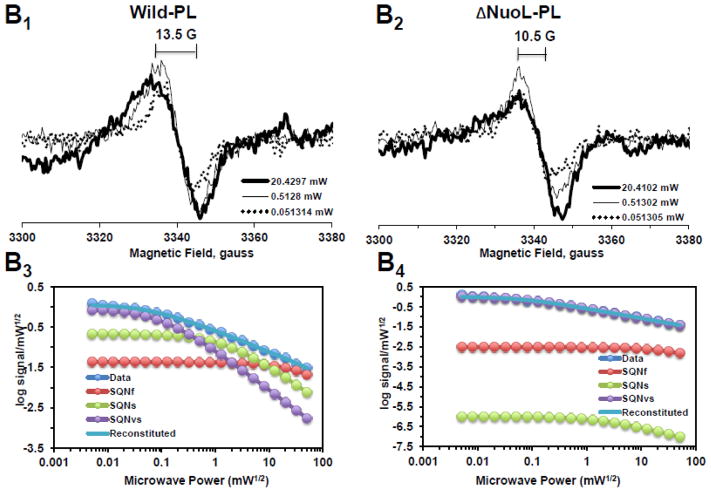
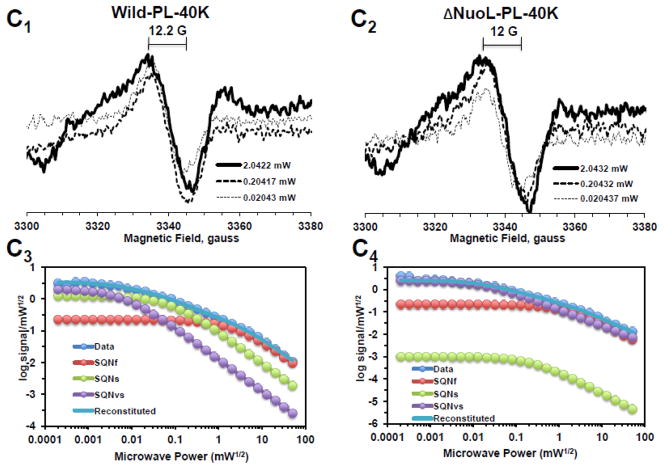
Fig. 2.
EPR spectra and the progressive power saturation profiles of ubisemiquinone g = 2.00 signals in the purified complex I at 150 K (A), the reconstituted complex I at 150 K (B) and 40 K (C). Panels A1, B1, and C1, or A2, B2, and C2 are EPR data for the wild-type or the ΔNuoL variant, respectively. A3, B3, and C3, or A4, B4, and C4, are the power saturation profiles of ubisemiquinones in the wild-type or the ΔNuoL variant, respectively. (A) The purified complex I (the wild-type, 8.28 mg/ml; the ΔNuoL variant,5.0 mg/ml) were anaerobically reduced with 6 mM NADH. The EPR data was analyzed as a three component system. “Data” and “Reconstituted” represent “actual EPR data” and “combined data of three resolved components after the fitting analysis”, respectively. The parameters obtained for the wild-type complex I are SQN1 (SQ species 1), C = 0.318; P1/2 = 3.84; b = 1.104; SQN2 (SQ species 2): C= 0.335; P1/2 = 0.164; b = 2; SQN3 (SQ species 3): C= 1.048; P1/2 = 0.002; b = 2. The parameters obtained for the ΔNuoL variant are SQN1: C = 0.662; P1/2 = 5.609; b = 1.211; SQN2: C= 0.261; P1/2 = 0.292; b = 1; SQN3: C= 0.667; P1/2 = 0.006; b = 1 (B) The reconstituted proteoliposomes were incubated on ice with 400 μM DQ, transferred into EPR tubes, and brought into an anaerobic chamber. The samples were anaerobically frozen at 10 sec after the addition of NADH to a final concentration of 2 mM. The parameters obtained for the wild-type are SQNf: C = 0.0426; P1/2 = 50; b = 2; SQNs: C = 0.2118; P1/2 = 1.624; b = 1.918; SQNvs: C = 0.8782; P1/2 = 0.1; b = 2. The parameters for the ΔNuoL variant are SQNf: C = 0.0030; P1/2 = 50; b = 2; SQNs: C = 0.0000; P1/2 = 2.0; b = 1.431; SQNvs: C = 1.0163; P1/2 = 0.1; b = 1. (C) The same proteoliposome samples described in (B) were used. The parameters for the wild-type are SQNf: C = 0.2213; P1/2 = 2.307; b = 2; SQNs: C = 1.242; P1/2 = 0.078; b = 2; SQNvs: C = 2.0012; P1/2 = 0.006; b = 2. The parameters for the ΔNuoL variant are SQNf: C = 0.2135; P1/2 = 1.405; b = 2; SQNs: C = 0.001; P1/2 = 0.229; b = 1.994; SQNvs: C = 2.4572; P1/2 = 0.017; b = 1.452. The gz signal of cluster N1a signal, which partially overlaps with the SQ signals at high microwave powers, was subtracted using the corresponding data obtained from their counter samples that were anaerobically reduced with 20 mM dithionite. Other EPR conditions were: microwave frequency, 9.45 GHz; modulation frequency, 100 kHz; modulation amplitude, 6 G; time constant, 82 ms. The concentrations and the specific activities of the proteoliposome samples are, Wild-type (WT)-proteoliposomes (PL): [1.8 mg/ml], NADH-DQ activity = 7.96 μmol/min/mg, NADH:ferricyanide activities = 78 μmol/min/mg; ΔNuoL-PL: [1.0 mg/ml], NADH-DQ activity = 2.38 μmol/min/mg, NADH:ferricyanide activities = 80.4 μmol/min/mg. The data shown in the figure are the representative data from three different samples for WT, WT-PL, and ΔNuoL-PL and two samples for ΔNuoL.