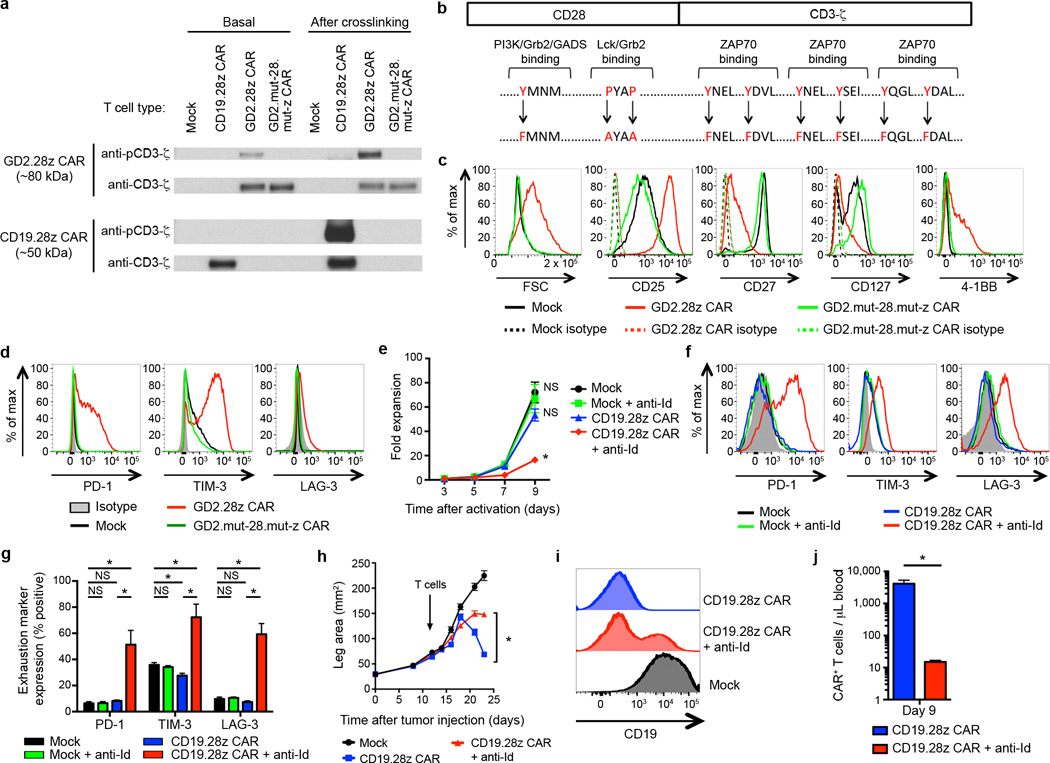
Figure 3.
Tonic CAR signaling during ex vivo expansion leads to early exhaustion. (a) Western blot evaluating phosphorylation levels of CAR signaling domains versus total CAR signaling domains, using an anti-phospho-CD3ζ and anti-CD3ζ antibody, respectively. Evaluated day 5 after initial activation. “Basal” phosphorylation evaluated without further stimulation. “After crosslinking” evaluated following incubation with OKT3, anti-CD19 CAR idiotype, or anti-GD2 CAR idiotype antibodies. Representative of 3 donors. (b) Nine amino acid point mutations were introduced to eliminate signaling via the GD2.28z CAR (GD2.mut-28.mut-z CAR). (c) Activation and (d) exhaustion marker expression of GD2.mut-28.mut-z CAR T cells 10 days after initial activation. Representative of 3 donors. (e) Ex vivo expansion and (f-g) exhaustion marker expression of CD19.28z CAR T cells cultured ± anti-idiotype antibody (anti-Id; 0.25 ug/ml) and a crosslinking F(ab’)2 (2.5 ug/ml). n=4 replicates/group; representative of 3 donors. (h) Tumor growth curves of NSG mice inoculated with 106 143B-CD19 periosteally on day 0 followed by adoptive transfer of 107 transduced CAR T cells on day 14. Mice received mock-transduced or CD19.28z CAR cultured with or without anti-idiotype antibody. No anti-idiotype antibody was given to mice. n=8 mice/group. (i) CD19 expression on tumors 10 days following adoptive transfer into mice from (h). (j) Quantification of T cells within the blood 9 days following adoptive T cell transfer into mice from (h). n=3 mice/group. In vivo results are representative of two experiments. * = p<0.05 by Student’s T-test. Bar graphs represent mean ± SEM.