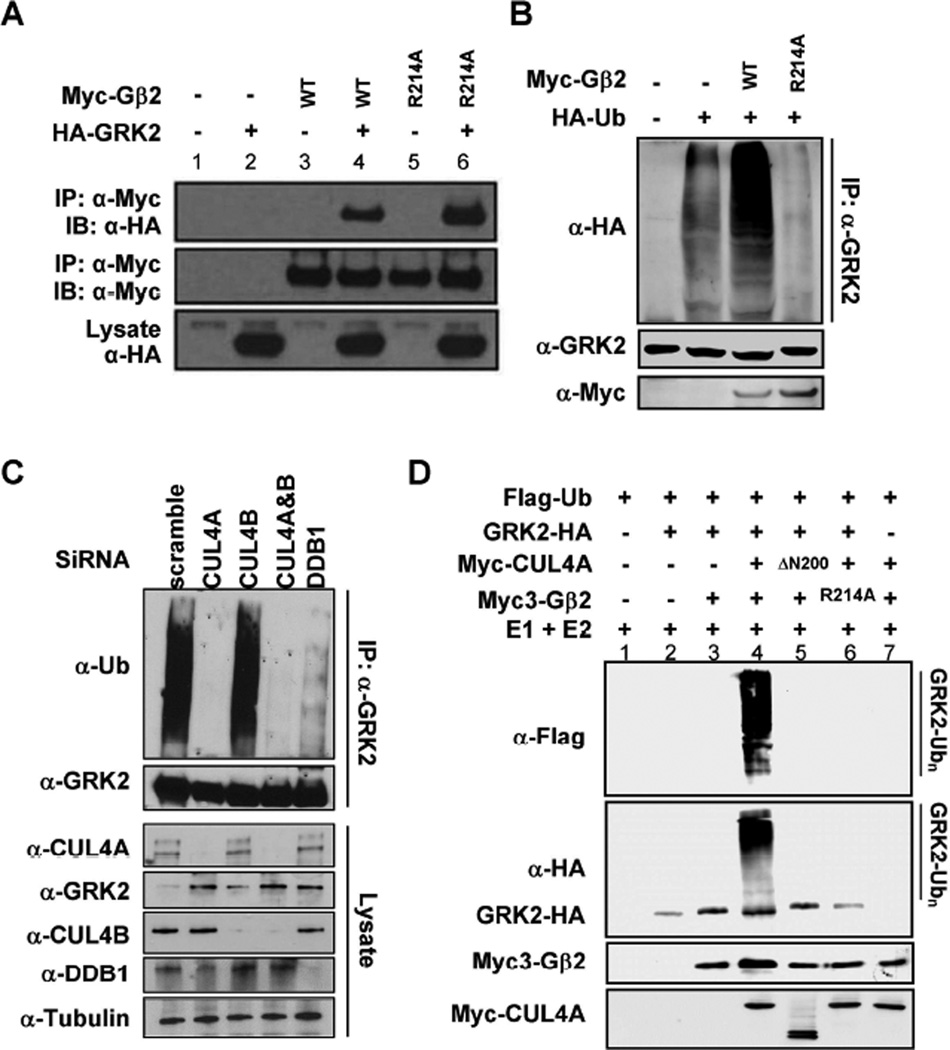
Figure 2. GRK2 is a substrate of CRL4Gβ2 E3 ubiquitin ligase.
(A) GRK2 binds to Gβ2. 293T cells were co-transfected with plasmids expressing indicated proteins and protein-protein interactions were determined by co-IP.
(B) Gβ2 promotes GRK2 ubiquitylation. HEK293 cells were transfected with plasmids expressing indicated proteins. Endogenous GRK2 was immunoprecipitated and analyzed for ubiquitylation by immunoblotting.
(C) Knocking down of CUL4A and DDB1 abolishes GRK2 ubiquitylation in vivo. HEK293 cells were transfected with siRNA oligonucleotides targeting indicated genes. The efficiency of knocking down was verified by immunoblotting. In vivo GRK2 ubiquitylation was determined by immunoprecipitation using an antibody specific to GRK2, followed by immunoblotting with an antibody specific to ubiquitin.
(D) In vitro ubiquitylation of GRK2 by CRL4Gβ2 E3 ligase. Purified GRK2 protein was incubated with CUL4A immunocomplex alone or with purified Gβ2 in the presence of E1, E2, ATP and ubiquitin. After termination, the reaction mixtures were resolved by SDS-PAGE, followed by immunoblotting with indicated antibodies.