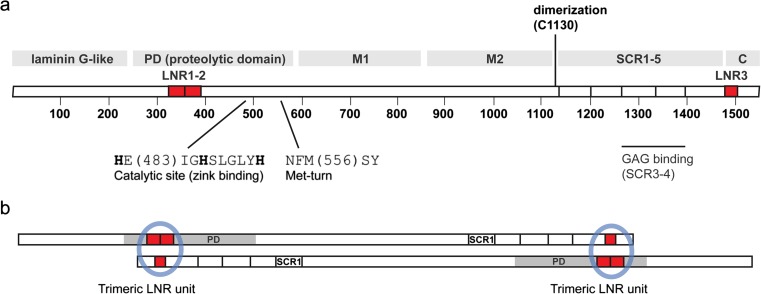
Fig. 1.
Schematic overview of the primary structure of PAPP-A. a The PAPP-A subunit is constituted by 1547 residues, 82 of which are cysteines. Protein modules are indicated with gray bars: The proteolytic domain (PD) of about 350 residues is preceded by a laminin G-like module of unknown function (Boldt et al. 2001). The PD contains the elongated zinc ion binding consensus sequence and a short sequence element responsible for formation of the Met-turn, both defining features of the metzincin superfamily of metalloproteinases (Boldt et al. 2001). Curiously, the second of the 22 exons of the PAPP-A gene encodes almost the entire laminin G-like module and approximately half of the PD (Overgaard et al. 2003). The PD is followed by two ill-defined regions, tentatively designated M1 and M2 based on disulfide structure, and then by five short consensus repeats (SCR1-5), also known as CCP modules (Overgaard et al. 2003). SCR3 and SCR4 bind glycosaminoglycans and are responsible for cell surface binding of PAPP-A (Laursen et al. 2002b). In addition, note that the PAPP-A subunit contains three Lin12-Notch repeat (LNR) modules shown in red, two of which (LNR1-2) are located within the PD and a third (LNR3) located in the C domain. The LNR modules determine proteolytic specificity of PAPP-A (Boldt et al. 2004). b PAPP-A exists as a 400 kDa dimer composed of two disulfide linked subunits. This simplified model highlights the antiparallel arrangement of the subunits and the two putative LNR units (blue circles), each formed from LNR1-2 in one subunit and LNR3 in the other (Weyer et al. 2007)