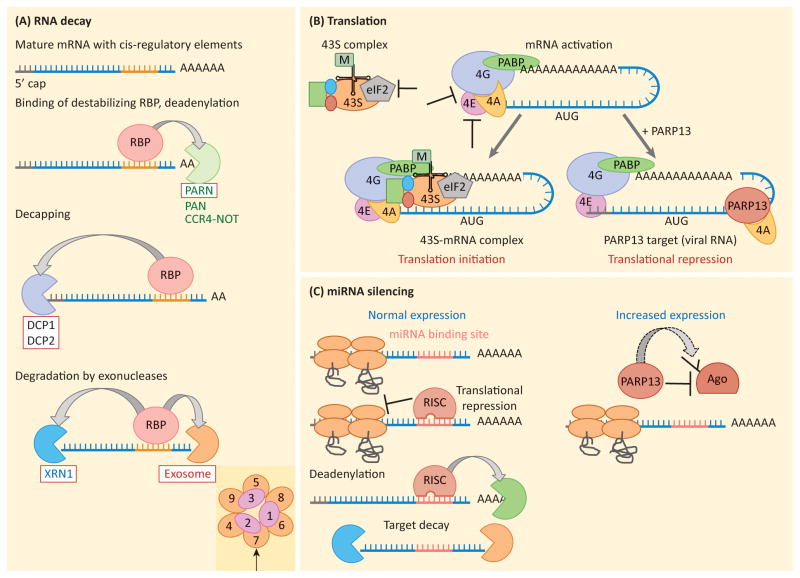
Figure 2. Mechanisms of RNA regulation.
a) RNA decay. Cis-regulatory elements found within an RNA sequence (usually within the 3′UTR) mediate binding of a destabilizing RNA-binding protein (RBP). The RBP can recruit specific RNA decay factors (arrows), but RNA decay usually initiates with removal of the poly(A) tail by deadenylases PAN, PARN or the CCR4-NOT complex. Removal of the poly(A) tail recruits decapping enzymes, including DCP1 and DCP2, which remove the 5′ methyl-guanine cap structure. The now unprotected RNA is degraded by the processive 5′-3′ exonuclease, XRN1, or 3′-5′ exonuclease, the exosome complex. Decay factors recruited specifically by PARP13 are boxed in red. An indent shows the structure of the exosome complex, with EXOSC1-EXOSC9 subunits identified. Arrow points to EXOSC7, the subunit that binds PARP13 in human.. b) Translation initiation and translational repression. Translation initiation begins by activating the mRNA via binding of the cap-binding factor eIF4E, the scaffold protein eIF4G and the helicase eIF4A. Interactions between eIF4G and PABP are required to stabilize the complex which then recruits the 43S complex, consisting of the small ribosomal subunit, loaded with the ternary complex (Met-tRNA and GTP bound eIF2), and additional initiation factors. The 43S-RNA complex is then competent for translation initiation, scanning and 60S subunit joining. T-bars show steps of translation initiation known to be inhibited by RNA-binding proteins. PARP13 represses translation by binding eIF4A and preventing it from interacting with eIF4G, a step required for cap-dependent translation initiation. c) miRNA silencing. Left, RISC complex loaded with miRNA silences its target by repressing translation and recruiting deadenylation factors, thus initiating decay. Right, PARP13 targets Argonaute (Ago), a component of the RISC complex, for poly(ADP-ribos)ylation by another PARP protein. Modification of Ago inhibits its function, likely by decreasing the binding affinity of the Ago-miRNA complex for the target mRNA. This results in global repression of miRNA silencing and increased expression of miRNA targets.