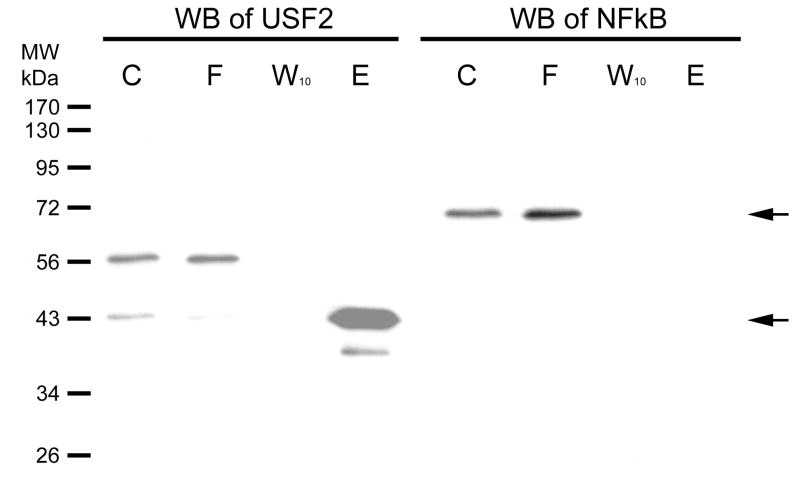
Figure 4. Western blot of USF-2 and a negative control on column fraction.
HEK293 nuclear extract was diluted 5-fold in TE0.1 and affinity chromatographic purification performed using SJ9 oligonucleotide (5′-GCTTCCCACGTGCGCArG-3′) annealed with the SJ11 complementary strand. The sequence is from the human telomerase (hTERT) promoter proximal E-box. Previously, we had shown that this sequence binds USF-2 [13]. The initial mixture (C), flow through (F), last wash through (W10) and eluate (E) by high salt were resolved by 12% SDS PAGE gel. Gels were electroblotted onto 0.2 μm PVDF membranes as previously described [14]. The dilution of USF2 (N-18) antibody was 1:1,000. Immunoreactive proteins were visualized using 1:10,000 goat anti-rabbit secondary antibody-HRP conjugate (Santa Cruz Biotechnology, CA) and detected by enhanced chemiluminescence (ECL). The Western blot of NFκB was done on the same blot after stripping, and the primary and secondary antibodies were diluted as 1:1,000 and 1:10,000, respectively. The arrows indicate where USF2 (~43kDa) or NFκB (~65 kDa) are located.