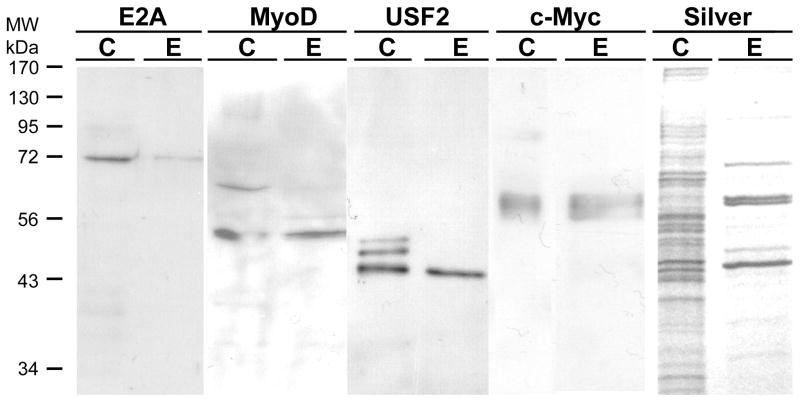
Figure 6. Other E-box binding transcription factors are also purified by the trapping method.
500 μg of freshly prepared HEK293 nuclear extract was prepared for trapping using 200 nM annealed SJ9/SJ11 duplex oligonucleotide in buffer KP. After incubation for 30 min on ice, the mixture was mixed with 100 μl of hydrazide agarose and allowed to couple at 4°C for 4 h on a rotating wheel mixer. The mixture was then packed into a column and the unretained (FT, flow-through) fraction saved. The column was washed with 0.5 ml of TE0.1 buffer five times and then eluted with 0.5 ml of TE1.5. The eluted fraction (E) was collected, concentrated in a centrifugal concentrator to 40 μl. 20 μl was loaded onto 12% SDS-PAGE along with 20 μl of 10 μg HEK293 nuclear extract (C) and 20 μl of the flow-through fraction (FT) for electrophoresis and electroblotting. The blots were then probed with an antibody against USF-2, E2A, c-Myc or MyoD as indicated in the figure. A duplicate gel was also stained with silver in a separate experiment.