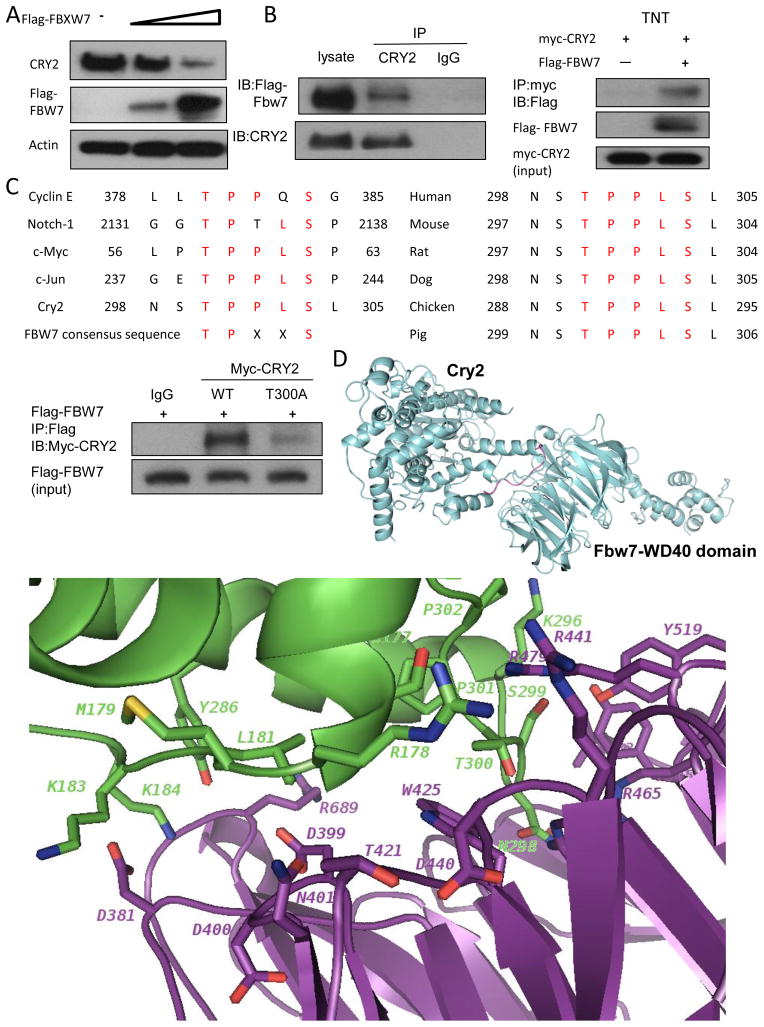
Figure 3. FBXW7 interacts with CRY2.
(A) FBXW7 negatively regulates the steady-state expression of CRY2. DLD-1 cells were transfected with indicated plasmids and increasing amounts of FBXW7. Equal amounts of cell lysates were immunoblotted with the indicated antibodies.
(B) Interaction of FBXW7 with CRY2. Equal amounts of DLD-1 cell lysates transfected with Flag-FBXW7 were immunoprecipitated with anti-CRY2 and immunoblotted with anti-Flag. FBXW7 interacts with CRY2 in vitro. Flag-FBXW7 and Myc-CRY2 cDNAs were transcribed and translated in vitro (TNT). FBXW7 and CRY2 proteins were incubated overnight and immunoprecipitated with anti-Myc followed by immunoblotting with anti-Flag.
(C) The consensus FBXW7 binding motif is highlighted. Sequences of CRY2 and other known FBXW7 substrates are shown for comparison (left panel). Sequence alignment of CRY2 containing FBXW7 binding motifs from different species is shown (right panel). Equal amounts of cell lysates from DLD-1 cotransfected with WT myc-CRY2 or myc-CRY2-T300A and Flag-FBXW7 plasmids were immunoprecipitated with anti-Flag and immunoblotted with indicated antibodies.
(D) Ribbon presentation of CRY2-FBXW7 complex model. The loop of CRY2 degron-motif (residues T300-S304) interacts with the WD40 domain of FBXW7. Close-up view of the interface in CRY2-FBXW7 complex. The interaction residues involving in the interface of CRY2-FBXW7 molecules were shown in stick.