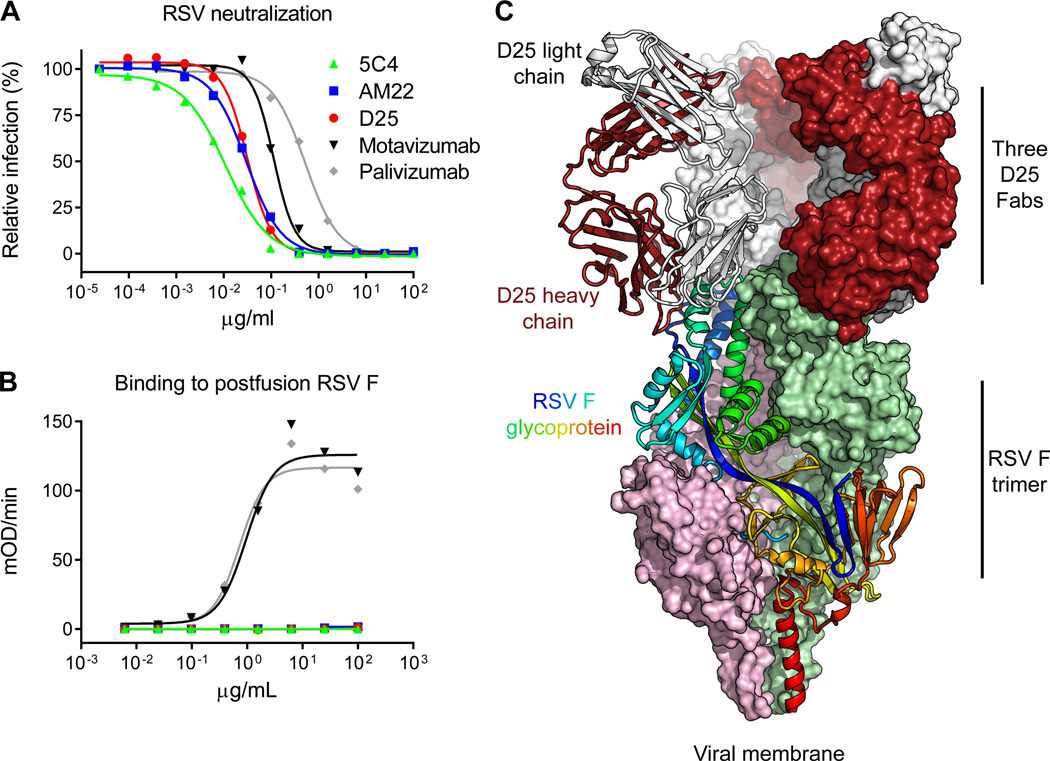
Figure 1. RSV neutralization, F glycoprotein recognition, and crystal structure of human antibody D25 in complex with the prefusion RSV F trimer.
The prefusion conformation of RSV F is metastable, and when expressed in a soluble form readily adopts the postfusion state; a number of potent antibodies, including D25, bind to a newly revealed antigenic site at the top of the prefusion F glycoprotein. (A) RSV neutralization by antibodies. Palivizumab is the FDA-approved prophylactic antibody that prevents severe RSV disease. (B) ELISA measuring antibody binding to postfusion F glycoprotein. For (A) and (B), data are representative of multiple independent experiments. (C) D25-RSV F trimer crystal structure in ribbon and molecular surface representations. One protomer of the F glycoprotein trimer is shown as ribbons and colored as a rainbow from blue to red, N-terminus of F2 to C-terminus of F1, respectively. Molecular surfaces are shown for the other two F protomers, colored pink and green. The D25 Fab bound to the F protomer shown in ribbons is also displayed in ribbon representation, with heavy chain colored red and light chain colored grey. The other D25 Fabs are colored the same, but shown in surface representation.