Abstract
Duchenne muscular dystrophy is a severe X chromosome-linked, muscle-wasting disease caused by lack of the protein dystrophin. The exact function of dystrophin remains to be determined. However, analysis of its interaction with a large oligomeric protein complex at the sarcolemma and the identification of a structurally related protein, utrophin, is leading to the characterization of candidate genes for other neuromuscular disorders.
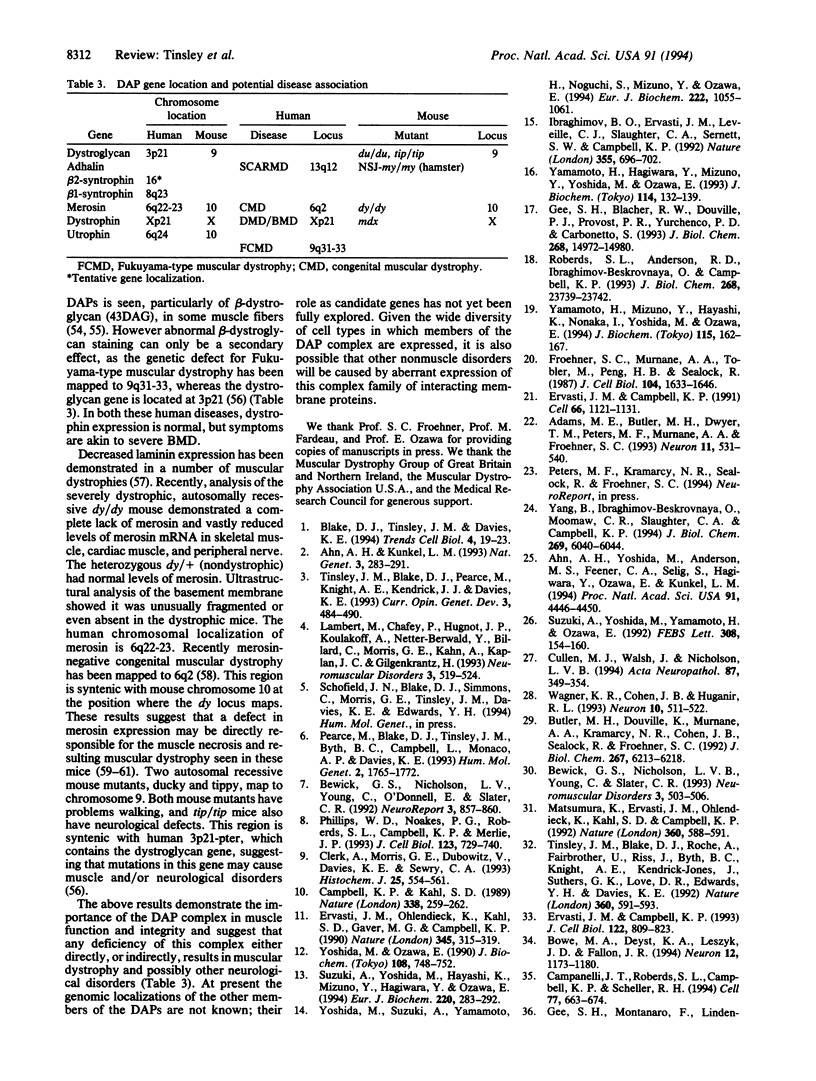
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. E., Butler M. H., Dwyer T. M., Peters M. F., Murnane A. A., Froehner S. C. Two forms of mouse syntrophin, a 58 kd dystrophin-associated protein, differ in primary structure and tissue distribution. Neuron. 1993 Sep;11(3):531–540. doi: 10.1016/0896-6273(93)90157-m. [DOI] [PubMed] [Google Scholar]
- Ahn A. H., Kunkel L. M. The structural and functional diversity of dystrophin. Nat Genet. 1993 Apr;3(4):283–291. doi: 10.1038/ng0493-283. [DOI] [PubMed] [Google Scholar]
- Ahn A. H., Yoshida M., Anderson M. S., Feener C. A., Selig S., Hagiwara Y., Ozawa E., Kunkel L. M. Cloning of human basic A1, a distinct 59-kDa dystrophin-associated protein encoded on chromosome 8q23-24. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4446–4450. doi: 10.1073/pnas.91.10.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arahata K., Hayashi Y. K., Mizuno Y., Yoshida M., Ozawa M. Dystrophin-associated glycoprotein and dystrophin co-localisation at sarcolemma in Fukuyama congenital muscular dystrophy. Lancet. 1993 Sep 4;342(8871):623–624. doi: 10.1016/0140-6736(93)91454-t. [DOI] [PubMed] [Google Scholar]
- Azibi K., Bachner L., Beckmann J. S., Matsumura K., Hamouda E., Chaouch M., Chaouch A., Ait-Ouarab R., Vignal A., Weissenbach J. Severe childhood autosomal recessive muscular dystrophy with the deficiency of the 50 kDa dystrophin-associated glycoprotein maps to chromosome 13q12. Hum Mol Genet. 1993 Sep;2(9):1423–1428. doi: 10.1093/hmg/2.9.1423. [DOI] [PubMed] [Google Scholar]
- Ben Othmane K., Ben Hamida M., Pericak-Vance M. A., Ben Hamida C., Blel S., Carter S. C., Bowcock A. M., Petruhkin K., Gilliam T. C., Roses A. D. Linkage of Tunisian autosomal recessive Duchenne-like muscular dystrophy to the pericentromeric region of chromosome 13q. Nat Genet. 1992 Dec;2(4):315–317. doi: 10.1038/ng1292-315. [DOI] [PubMed] [Google Scholar]
- Bewick G. S., Nicholson L. V., Young C., O'Donnell E., Slater C. R. Different distributions of dystrophin and related proteins at nerve-muscle junctions. Neuroreport. 1992 Oct;3(10):857–860. doi: 10.1097/00001756-199210000-00009. [DOI] [PubMed] [Google Scholar]
- Bewick G. S., Nicholson L. V., Young C., Slater C. R. Relationship of a dystrophin-associated glycoprotein to junctional acetylcholine receptor clusters in rat skeletal muscle. Neuromuscul Disord. 1993 Sep-Nov;3(5-6):503–506. doi: 10.1016/0960-8966(93)90105-s. [DOI] [PubMed] [Google Scholar]
- Blake D. J., Tinsley J. M., Davies K. E. The emerging family of dystrophin-related proteins. Trends Cell Biol. 1994 Jan;4(1):19–23. doi: 10.1016/0962-8924(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Bowe M. A., Deyst K. A., Leszyk J. D., Fallon J. R. Identification and purification of an agrin receptor from Torpedo postsynaptic membranes: a heteromeric complex related to the dystroglycans. Neuron. 1994 May;12(5):1173–1180. doi: 10.1016/0896-6273(94)90324-7. [DOI] [PubMed] [Google Scholar]
- Butler M. H., Douville K., Murnane A. A., Kramarcy N. R., Cohen J. B., Sealock R., Froehner S. C. Association of the Mr 58,000 postsynaptic protein of electric tissue with Torpedo dystrophin and the Mr 87,000 postsynaptic protein. J Biol Chem. 1992 Mar 25;267(9):6213–6218. [PubMed] [Google Scholar]
- Campanelli J. T., Roberds S. L., Campbell K. P., Scheller R. H. A role for dystrophin-associated glycoproteins and utrophin in agrin-induced AChR clustering. Cell. 1994 Jun 3;77(5):663–674. doi: 10.1016/0092-8674(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Campbell K. P., Kahl S. D. Association of dystrophin and an integral membrane glycoprotein. Nature. 1989 Mar 16;338(6212):259–262. doi: 10.1038/338259a0. [DOI] [PubMed] [Google Scholar]
- Clerk A., Morris G. E., Dubowitz V., Davies K. E., Sewry C. A. Dystrophin-related protein, utrophin, in normal and dystrophic human fetal skeletal muscle. Histochem J. 1993 Aug;25(8):554–561. [PubMed] [Google Scholar]
- Cullen M. J., Walsh J., Nicholson L. V. Immunogold localization of the 43-kDa dystroglycan at the plasma membrane in control and dystrophic human muscle. Acta Neuropathol. 1994;87(4):349–354. doi: 10.1007/BF00313603. [DOI] [PubMed] [Google Scholar]
- Ervasti J. M., Campbell K. P. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol. 1993 Aug;122(4):809–823. doi: 10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervasti J. M., Campbell K. P. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991 Sep 20;66(6):1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- Ervasti J. M., Ohlendieck K., Kahl S. D., Gaver M. G., Campbell K. P. Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature. 1990 May 24;345(6273):315–319. doi: 10.1038/345315a0. [DOI] [PubMed] [Google Scholar]
- Fardeau M., Matsumura K., Tomé F. M., Collin H., Leturcq F., Kaplan J. C., Campbell K. P. Deficiency of the 50 kDa dystrophin associated glycoprotein (adhalin) in severe autosomal recessive muscular dystrophies in children native from European countries. C R Acad Sci III. 1993 Aug;316(8):799–804. [PubMed] [Google Scholar]
- Froehner S. C., Murnane A. A., Tobler M., Peng H. B., Sealock R. A postsynaptic Mr 58,000 (58K) protein concentrated at acetylcholine receptor-rich sites in Torpedo electroplaques and skeletal muscle. J Cell Biol. 1987 Jun;104(6):1633–1646. doi: 10.1083/jcb.104.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee S. H., Blacher R. W., Douville P. J., Provost P. R., Yurchenco P. D., Carbonetto S. Laminin-binding protein 120 from brain is closely related to the dystrophin-associated glycoprotein, dystroglycan, and binds with high affinity to the major heparin binding domain of laminin. J Biol Chem. 1993 Jul 15;268(20):14972–14980. [PubMed] [Google Scholar]
- Gee S. H., Montanaro F., Lindenbaum M. H., Carbonetto S. Dystroglycan-alpha, a dystrophin-associated glycoprotein, is a functional agrin receptor. Cell. 1994 Jun 3;77(5):675–686. doi: 10.1016/0092-8674(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Hayashi Y. K., Engvall E., Arikawa-Hirasawa E., Goto K., Koga R., Nonaka I., Sugita H., Arahata K. Abnormal localization of laminin subunits in muscular dystrophies. J Neurol Sci. 1993 Oct;119(1):53–64. doi: 10.1016/0022-510x(93)90191-z. [DOI] [PubMed] [Google Scholar]
- Helliwell T. R., Ellis J. M., Mountford R. C., Appleton R. E., Morris G. E. A truncated dystrophin lacking the C-terminal domains is localized at the muscle membrane. Am J Hum Genet. 1992 Mar;50(3):508–514. [PMC free article] [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya O., Ervasti J. M., Leveille C. J., Slaughter C. A., Sernett S. W., Campbell K. P. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992 Feb 20;355(6362):696–702. doi: 10.1038/355696a0. [DOI] [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya O., Milatovich A., Ozcelik T., Yang B., Koepnick K., Francke U., Campbell K. P. Human dystroglycan: skeletal muscle cDNA, genomic structure, origin of tissue specific isoforms and chromosomal localization. Hum Mol Genet. 1993 Oct;2(10):1651–1657. doi: 10.1093/hmg/2.10.1651. [DOI] [PubMed] [Google Scholar]
- Kramarcy N. R., Vidal A., Froehner S. C., Sealock R. Association of utrophin and multiple dystrophin short forms with the mammalian M(r) 58,000 dystrophin-associated protein (syntrophin). J Biol Chem. 1994 Jan 28;269(4):2870–2876. [PubMed] [Google Scholar]
- Lambert M., Chafey P., Hugnot J. P., Koulakoff A., Berwald-Netter Y., Billard C., Morris G. E., Kahn A., Kaplan J. C., Gilgenkrantz H. Expression of the transcripts initiated in the 62nd intron of the dystrophin gene. Neuromuscul Disord. 1993 Sep-Nov;3(5-6):519–524. doi: 10.1016/0960-8966(93)90108-v. [DOI] [PubMed] [Google Scholar]
- Matsumura K., Burghes A. H., Mora M., Tomé F. M., Morandi L., Cornello F., Leturcq F., Jeanpierre M., Kaplan J. C., Reinert P. Immunohistochemical analysis of dystrophin-associated proteins in Becker/Duchenne muscular dystrophy with huge in-frame deletions in the NH2-terminal and rod domains of dystrophin. J Clin Invest. 1994 Jan;93(1):99–105. doi: 10.1172/JCI116989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura K., Ervasti J. M., Ohlendieck K., Kahl S. D., Campbell K. P. Association of dystrophin-related protein with dystrophin-associated proteins in mdx mouse muscle. Nature. 1992 Dec 10;360(6404):588–591. doi: 10.1038/360588a0. [DOI] [PubMed] [Google Scholar]
- Matsumura K., Nonaka I., Arahata K., Campbell K. P. Partial deficiency of dystrophin-associated proteins in a young girl with sporadic myopathy and normal karyotype. Neurology. 1993 Jun;43(6):1267–1268. doi: 10.1212/wnl.43.6.1267. [DOI] [PubMed] [Google Scholar]
- Matsumura K., Nonaka I., Campbell K. P. Abnormal expression of dystrophin-associated proteins in Fukuyama-type congenital muscular dystrophy. Lancet. 1993 Feb 27;341(8844):521–522. doi: 10.1016/0140-6736(93)90279-p. [DOI] [PubMed] [Google Scholar]
- Matsumura K., Nonaka I., Tomé F. M., Arahata K., Collin H., Leturcq F., Récan D., Kaplan J. C., Fardeau M., Campbell K. P. Mild deficiency of dystrophin-associated proteins in Becker muscular dystrophy patients having in-frame deletions in the rod domain of dystrophin. Am J Hum Genet. 1993 Aug;53(2):409–416. [PMC free article] [PubMed] [Google Scholar]
- Matsumura K., Tomé F. M., Collin H., Azibi K., Chaouch M., Kaplan J. C., Fardeau M., Campbell K. P. Deficiency of the 50K dystrophin-associated glycoprotein in severe childhood autosomal recessive muscular dystrophy. Nature. 1992 Sep 24;359(6393):320–322. doi: 10.1038/359320a0. [DOI] [PubMed] [Google Scholar]
- Matsumura K., Tomé F. M., Collin H., Leturcq F., Jeanpierre M., Kaplan J. C., Fardeau M., Campbell K. P. Expression of dystrophin-associated proteins in dystrophin-positive muscle fibers (revertants) in Duchenne muscular dystrophy. Neuromuscul Disord. 1994 Mar;4(2):115–120. doi: 10.1016/0960-8966(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Matsumura K., Tomé F. M., Ionasescu V., Ervasti J. M., Anderson R. D., Romero N. B., Simon D., Récan D., Kaplan J. C., Fardeau M. Deficiency of dystrophin-associated proteins in Duchenne muscular dystrophy patients lacking COOH-terminal domains of dystrophin. J Clin Invest. 1993 Aug;92(2):866–871. doi: 10.1172/JCI116661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno Y., Yoshida M., Nonaka I., Hirai S., Ozawa E. Expression of utrophin (dystrophin-related protein) and dystrophin-associated glycoproteins in muscles from patients with Duchenne muscular dystrophy. Muscle Nerve. 1994 Feb;17(2):206–216. doi: 10.1002/mus.880170212. [DOI] [PubMed] [Google Scholar]
- Ohlendieck K., Matsumura K., Ionasescu V. V., Towbin J. A., Bosch E. P., Weinstein S. L., Sernett S. W., Campbell K. P. Duchenne muscular dystrophy: deficiency of dystrophin-associated proteins in the sarcolemma. Neurology. 1993 Apr;43(4):795–800. doi: 10.1212/wnl.43.4.795. [DOI] [PubMed] [Google Scholar]
- Passos-Bueno M. R., Oliveira J. R., Bakker E., Anderson R. D., Marie S. K., Vainzof M., Roberds S., Campbell K. P., Zatz M. Genetic heterogeneity for Duchenne-like muscular dystrophy (DLMD) based on linkage and 50 DAG analysis. Hum Mol Genet. 1993 Nov;2(11):1945–1947. doi: 10.1093/hmg/2.11.1945. [DOI] [PubMed] [Google Scholar]
- Pearce M., Blake D. J., Tinsley J. M., Byth B. C., Campbell L., Monaco A. P., Davies K. E. The utrophin and dystrophin genes share similarities in genomic structure. Hum Mol Genet. 1993 Nov;2(11):1765–1772. doi: 10.1093/hmg/2.11.1765. [DOI] [PubMed] [Google Scholar]
- Phillips W. D., Noakes P. G., Roberds S. L., Campbell K. P., Merlie J. P. Clustering and immobilization of acetylcholine receptors by the 43-kD protein: a possible role for dystrophin-related protein. J Cell Biol. 1993 Nov;123(3):729–740. doi: 10.1083/jcb.123.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberds S. L., Anderson R. D., Ibraghimov-Beskrovnaya O., Campbell K. P. Primary structure and muscle-specific expression of the 50-kDa dystrophin-associated glycoprotein (adhalin). J Biol Chem. 1993 Nov 15;268(32):23739–23742. [PubMed] [Google Scholar]
- Roberds S. L., Ervasti J. M., Anderson R. D., Ohlendieck K., Kahl S. D., Zoloto D., Campbell K. P. Disruption of the dystrophin-glycoprotein complex in the cardiomyopathic hamster. J Biol Chem. 1993 Jun 5;268(16):11496–11499. [PubMed] [Google Scholar]
- Sewry C. A., Sansome A., Matsumura K., Campbell K. P., Dubowitz V. Deficiency of the 50 kDa dystrophin-associated glycoprotein and abnormal expression of utrophin in two south Asian cousins with variable expression of severe childhood autosomal recessive muscular dystrophy. Neuromuscul Disord. 1994 Mar;4(2):121–129. doi: 10.1016/0960-8966(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Sunada Y., Bernier S. M., Kozak C. A., Yamada Y., Campbell K. P. Deficiency of merosin in dystrophic dy mice and genetic linkage of laminin M chain gene to dy locus. J Biol Chem. 1994 May 13;269(19):13729–13732. [PubMed] [Google Scholar]
- Suzuki A., Yoshida M., Hayashi K., Mizuno Y., Hagiwara Y., Ozawa E. Molecular organization at the glycoprotein-complex-binding site of dystrophin. Three dystrophin-associated proteins bind directly to the carboxy-terminal portion of dystrophin. Eur J Biochem. 1994 Mar 1;220(2):283–292. doi: 10.1111/j.1432-1033.1994.tb18624.x. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Yoshida M., Yamamoto H., Ozawa E. Glycoprotein-binding site of dystrophin is confined to the cysteine-rich domain and the first half of the carboxy-terminal domain. FEBS Lett. 1992 Aug 17;308(2):154–160. doi: 10.1016/0014-5793(92)81265-n. [DOI] [PubMed] [Google Scholar]
- Tinsley J. M., Blake D. J., Pearce M., Knight A. E., Kendrick-Jones J., Davies K. E. Dystrophin and related proteins. Curr Opin Genet Dev. 1993 Jun;3(3):484–490. doi: 10.1016/0959-437x(93)90124-8. [DOI] [PubMed] [Google Scholar]
- Tinsley J. M., Blake D. J., Roche A., Fairbrother U., Riss J., Byth B. C., Knight A. E., Kendrick-Jones J., Suthers G. K., Love D. R. Primary structure of dystrophin-related protein. Nature. 1992 Dec 10;360(6404):591–593. doi: 10.1038/360591a0. [DOI] [PubMed] [Google Scholar]
- Wagner K. R., Cohen J. B., Huganir R. L. The 87K postsynaptic membrane protein from Torpedo is a protein-tyrosine kinase substrate homologous to dystrophin. Neuron. 1993 Mar;10(3):511–522. doi: 10.1016/0896-6273(93)90338-r. [DOI] [PubMed] [Google Scholar]
- Xu H., Christmas P., Wu X. R., Wewer U. M., Engvall E. Defective muscle basement membrane and lack of M-laminin in the dystrophic dy/dy mouse. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5572–5576. doi: 10.1073/pnas.91.12.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H., Hagiwara Y., Mizuno Y., Yoshida M., Ozawa E. Heterogeneity of dystrophin-associated proteins. J Biochem. 1993 Jul;114(1):132–139. doi: 10.1093/oxfordjournals.jbchem.a124128. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Mizuno Y., Hayashi K., Nonaka I., Yoshida M., Ozawa E. Expression of dystrophin-associated protein 35DAG (A4) and 50DAG (A2) is confined to striated muscles. J Biochem. 1994 Jan;115(1):162–167. doi: 10.1093/oxfordjournals.jbchem.a124294. [DOI] [PubMed] [Google Scholar]
- Yamanouchi Y., Mizuno Y., Yamamoto H., Takemitsu M., Yoshida M., Nonaka I., Ozawa E. Selective defect in dystrophin-associated glycoproteins 50DAG (A2) and 35DAG (A4) in the dystrophic hamster: an animal model for severe childhood autosomal recessive muscular dystrophy (SCARMD). Neuromuscul Disord. 1994 Jan;4(1):49–54. doi: 10.1016/0960-8966(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Yang B., Ibraghimov-Beskrovnaya O., Moomaw C. R., Slaughter C. A., Campbell K. P. Heterogeneity of the 59-kDa dystrophin-associated protein revealed by cDNA cloning and expression. J Biol Chem. 1994 Feb 25;269(8):6040–6044. [PubMed] [Google Scholar]
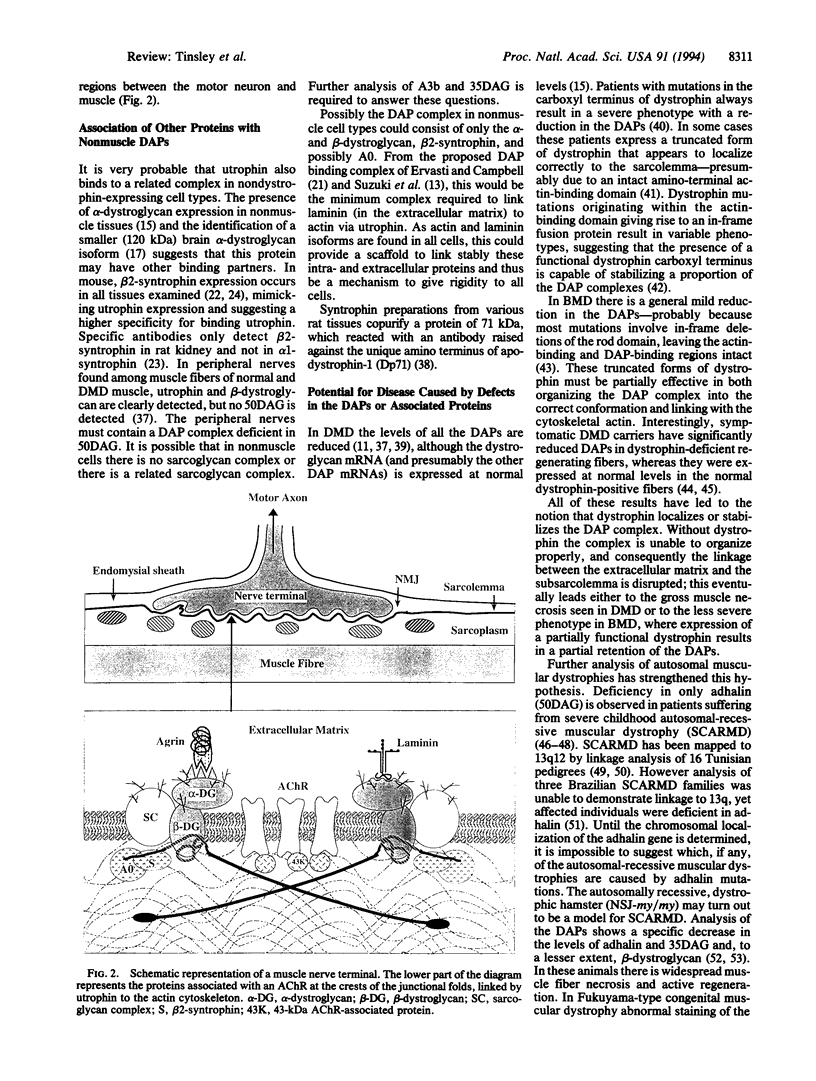
- Yoshida M., Ozawa E. Glycoprotein complex anchoring dystrophin to sarcolemma. J Biochem. 1990 Nov;108(5):748–752. doi: 10.1093/oxfordjournals.jbchem.a123276. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Suzuki A., Yamamoto H., Noguchi S., Mizuno Y., Ozawa E. Dissociation of the complex of dystrophin and its associated proteins into several unique groups by n-octyl beta-D-glucoside. Eur J Biochem. 1994 Jun 15;222(3):1055–1061. doi: 10.1111/j.1432-1033.1994.tb18958.x. [DOI] [PubMed] [Google Scholar]