Fig. 3.
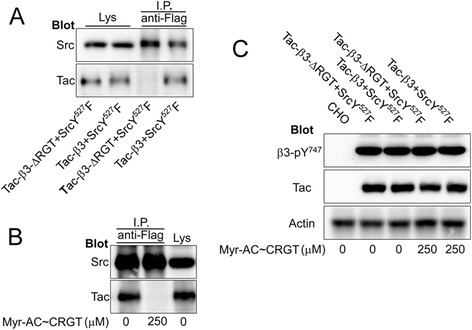
Disruption of the constitutive Src-β3 association did not affect the tyrosine phosphorylation of the β3 tail by active c-Src. a Lysates of the CHO cells expressing both Tac-β3 (or Tac-β3-ΔRGT) and c-Src Y527F were immunoprecipitated with anti-Flag antibody (for c-Src Y527F) and the immune complexes were subjected to SDS-PAGE and blotted with an anti-Tac monoclonal antibody. Tac-β3 but not Tac-β3-ΔRGT was co-immunoprecipitated with c-Src Y527F. b Lysates of CHO cells co-expressing Tac-β3 and c-Src Y527F pretreated with 250 μM of myr-AC ~ CRGT were immunoprecipitated by an anti-Flag antibody. The association of Src Y527F with β3 was disrupted by myr-AC ~ CRGT peptide. c Lysates of CHO cells co-expressing Tac-β3 (or Tac-β3-ΔRGT) and c-Src Y527F pretreated with 250 μM of myr-AC ~ CRGT were analyzed by Western blot using anti-β3-pY747 and anti-Tac antibodies. Myr-AC ~ CRGT did not affect the tyrosine phosphorylation of the β3 tail no matter whether it contains the RGT sequences. Actin served as a loading control
