Figure 1. Schematic diagram illustrating the Kiss1-GnRH connection and signaling pathways responsible for kisspeptin-induced depolarization and burst firing of GnRH neurons.
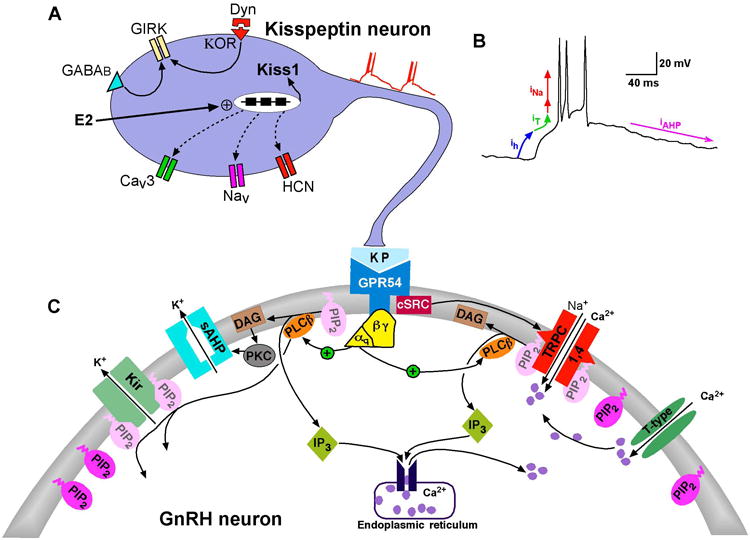
A. Kisspeptin neurons express the conductances (h-, T- and NaP) and corresponding channels (HCN, CaV3 and NaV) that generate burst firing necessary for maximum release of kisspeptin from the nerve terminal. E2 treatment increases the mRNA expression of Kiss1. In addition, the critical currents (Ih and IT.) are increased by circulating E2. The respective ion channel transcripts (HCN and CaV3, as well as NaV) are also expressed in Kiss1 neurons (dashed lines). E2 regulation of the mRNA expression of these channels is under investigation (manuscript in preparation). Kiss1 neurons also express GABAB and κ-opioid receptors, which couple to GIRK channels. B. Whole-cell current-clamp recording of spontaneous rebound burst firing recorded from an AVPV/PeN Kiss1 neuron. Arrows indicate the approximate range where the h-current (Ih, blue) and T-type calcium current (IT, green) and persistent Na current (INa, first red arrow) are active to depolarize the cell to threshold for generating an ensemble of fast Na+ spikes (INa, second red arrow) and AHP currents (IAHP, magenta) to repolarize the membrane to a hyperpolarized state to reinitiate the burst firing. The INa is composed of an initial INaP followed by an INaT (transient Na current). C. Kisspeptin binds to the Gq-coupled GPR54 receptor to activate phospholipase Cβ (PLCβ), which catabolizes PIP2, potentiates TRPC channel activity and inhibits the Kir channel activity. PKC, activated by the PIP2 hydrolysis product diacylglycerol (DAG), inhibits the activity of a calcium-activated slow afterhyperpolarization (sAHP) current. The non-receptor tyrosine (cSRC) kinase, which is activated by kisspeptin/GRP54 signaling, potentiates the activity of TRPC4 channels. Calcium entering the cell via T-type channels facilitates the activation of TRPC channels.
