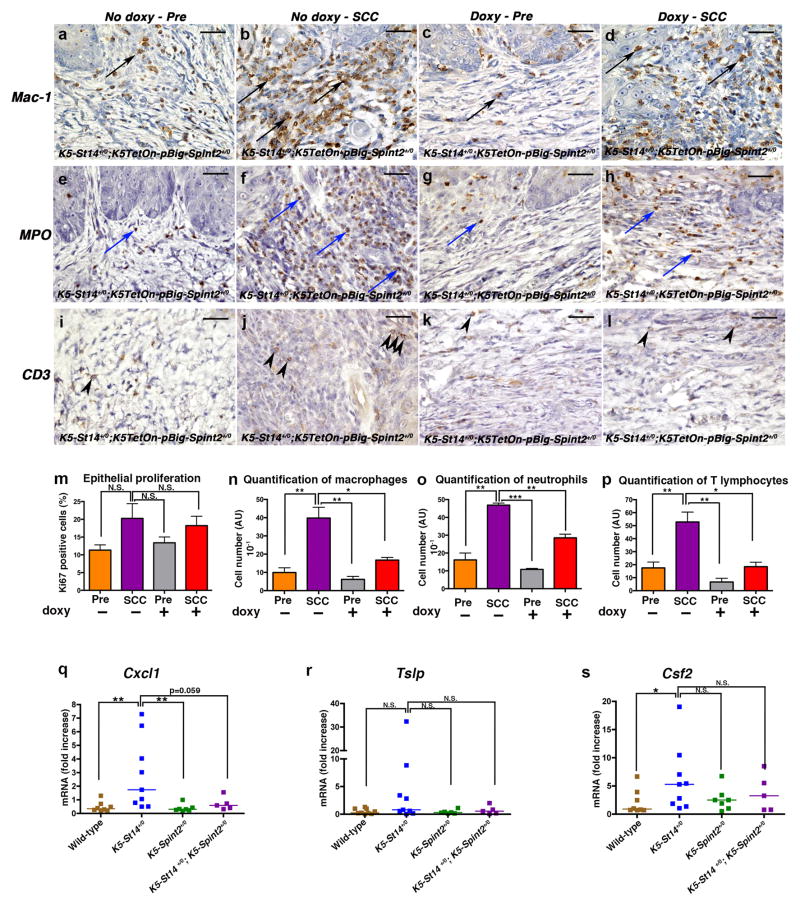
Figure 4. Inhibition of matriptase decreases inflammatory cell infiltration into established epidermal tumors.
(a–p) Immunohistochemical analysis of DMBA-treated K5-St14+/0; K5TetOn+/0; pBig-HAI-2+/0 triple-transgenic mice treated with 5 doses of 250 μg/ml DMBA and fed either control chow or fed doxycycline-containing chow for 14 weeks to induce HAI-2 expression in K5-expressing cells. (a–l) Representative immunohistochemistry sections of hyperplastic epithelia and SCC lesions showing macrophages (Mac1, a–d, black arrows), neutrophils (MPO, e–h, blue arrows) and T lymphocytes (CD3, i–l, arrowheads). Increased recruitment of inflammatory cells is present in SCC lesions from mice fed normal chow as compared to mice fed doxycycline-containing chow. Bar sizes: 100 μm. (m–p) Immunohistochemical analysis of DMBA-treated K5-St14+/0; K5TetOn+/0; pBig-HAI-2+/0 triple-transgenic mice fed either control chow (n=3, orange and purple bars) or fed doxycycline-containing chow for 14 weeks (n=3, grey and red bars). Samples from hyperplastic skin adjacent to SCC lesions (Pre, orange and grey bars), and Squamous Cell Carcinoma (SCC, purple and red bars) were analyzed. (m) Enumeration of Ki-67-positive cells was used as a marker for cell proliferation. No statistical difference in tumor cell proliferation was observed amongst groups. P=N.S., SCC fed control chow versus all the other groups (Student’s t-test, two-tailed). (n–p) Quantification of inflammatory cell infiltrates in hyperplastic skin and SCC, as described above, by enumeration of macrophages (n, Mac1 [Cd11b] antibodies), neutrophils (o, MPO antibodies) and T lymphocytes (p, CD3 antibodies). Densities of macrophages, neutrophils and T lymphocytes were significantly increased in tumors from mice that were fed normal chow when compared to mice fed doxycycline-containing chow to induce HAI-2 expression. *P<0.0198 and **P<0.0097 in n, **P<0.0016 and ***P<0.0001 in o, *P<0.0163 and **P<0.0048 in p, SCC fed control chow versus other groups (unpaired Student’s t-test, two-tailed). Skin and tumor tissues were fixed for 24 h in 4% paraformaldehyde in phosphate buffered saline, processed into paraffin, cut into sagittal 3-μm sections. Immunohistochemistry was performed using the following primary antibodies, anti-Ki-67 (Dako, Carpinteria, CA), anti-Mac1 [Cd11b] (Abcam, Cambridge, MA), anti-Myeloperoxidase (Abcam) and anti-CD3 (Abcam). After incubation with secondary antibodies, slides were developed using the diaminobenzidine substrate (Sigma-Aldrich), mounted and scanned using Scan Scope. (q–s) RNA was isolated from epidermis of 1-day-old wild type (brown squares, n=7), K5-St14+/0 (blue squares, n=9), K5-Spint2+/0 (green squares, n=7) and K5-St14+/0; K5-Spint2+/0 (purple squares, n=5) mice. Quantification of Cxcl-1 (q), Tslp (r), and Csf2 (s) mRNA levels were performed by real-time PCR using TaqMan probes. Cxcl-1, Tslp and Csf2 mRNA were all increased in the K5-St14+/0 mice when compared to K5-St14+/0; K5-Spint2+/0; K5-Spint2+/0 and wildtype littermates. **P<0.0025 in Q, P=N.S. in R, *P=0.0274 and P=N.S. in S, K5-St14+/0 versus the other genotypes (Mann-Whitney U test, two-tailed). Reverse transcription was performed using the High-Capacity cDNA Reverse Transcription Kit (Life Technologies), as recommended by the manufacturer. Cxcl1, Tslp and Csf2q PCRs were done using TaqMan probes following the manufacturer’s instructions (Life Technologies).