Fig. 3.
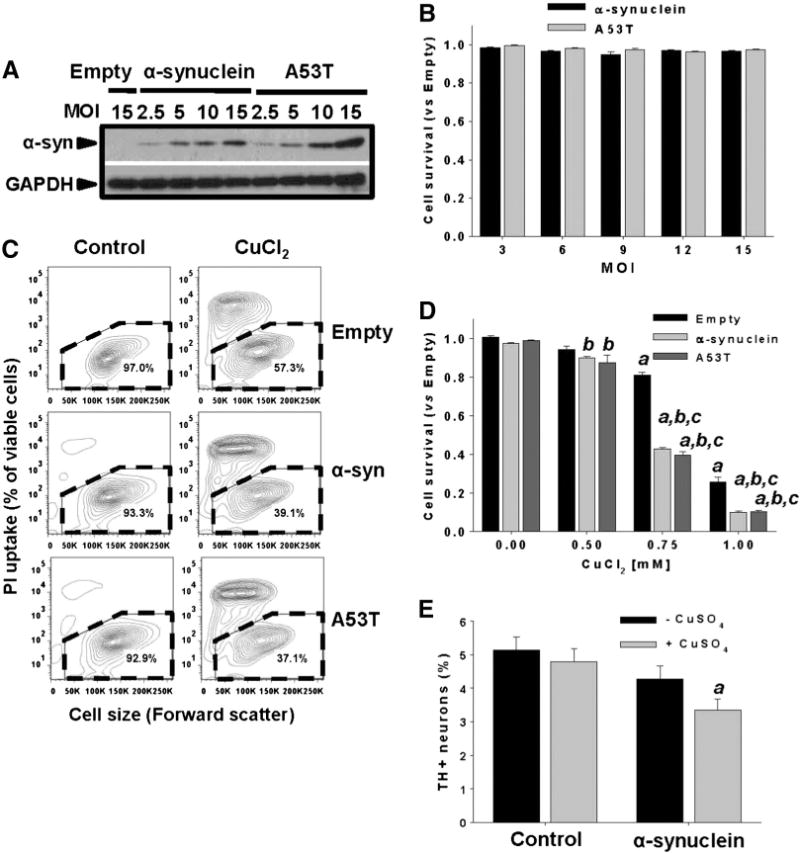
Non-toxic overexpression of α-synuclein sensitizes dopaminergic neuroblastoma cells and primary midbrain cultures to Cu toxicity. In A and B, neuroblastoma cells were transduced with Ad-Empty, Ad-α-synuclein or Ad-α-synuclein A53T mutant (A53T) at the indicated MOI for 24 h. Cells were washed, and the expression levels of WT or A53T α-synuclein were measured by WB after 48 h of transduction (A), while cell survival was evaluated by Calcein retention (B). In C and D, cell death induced by CuCl2 treatment (48 h, 0.75 mM in C) was determined in neuroblastoma cells previously transduced with WT or A53T α-synuclein using PI uptake (C) or Calcein retention assay (D). In C, cell death is represented in two-dimensional 5% probability contour plots of PI fluorescence vs cell size (forward scatter) to depict cells with both compromised plasma membrane integrity (PI uptake) and decreased cell size. %s in contour plots represent the number of viable cells (PI- and normal cell size, broken line regions). Data in B and D represent means ± SE of n = 3. In E, primary midbrain cultures transduced, or not, with WT α-synuclein (MOI = 10, 72 h) were incubated in fresh media with or without CuSO4 (10 μM) for 24 h. Cells were stained with antibodies specific for MAP2 and TH and scored for dopaminergic cell viability. The data are presented as the mean ± SE of n = 7. Two-way ANOVA, Holm-Sidak post hoc test, ap < 0.05 vs Empty without CuCl2 or CuSO4 supplementation; bp < 0.05 vs 0 mM CuCl2 within the corresponding category of α-synuclein or A53T; cp < 0.05 vs Empty within the corresponding [CuCl2] tested.
