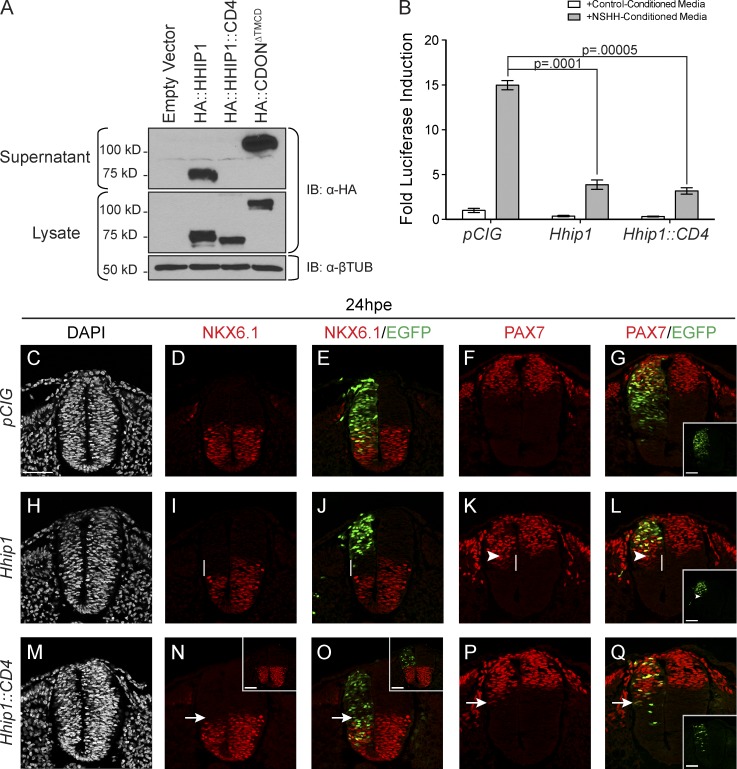
Figure 3.
HHIP1 secretion mediates its non–cell-autonomous effects in the developing neural tube. (A) Western blot analysis of cell lysates (bottom) and supernatants (top) collected from NIH/3T3 fibroblasts expressing HA-tagged HHIP1 proteins and probed with an anti-HA antibody. HA::CDONΔTMCD is included as a secreted protein control. Anti–β-tubulin (βTUB) is used as a loading control. (B) HH-responsive luciferase reporter activity measured in NIH/3T3 cells stimulated with either control-conditioned media or NSHH-conditioned media and transfected with the indicated constructs. Data are presented as means ± SEM. P-values are determined by two-tailed Student’s t tests. (C–Q) Neural patterning analysis of chicken embryos electroporated at stage 11–13 with pCIG (C–G), mHhip1-pCIG (H–L), and mHhip1::CD4-pCIG (M–Q) and collected at 24 hpe. Embryos were sectioned at the wing axial level and stained with antibodies against NKX6.1 (D, E, I, J, N, and O) and PAX7 (F, G, K, L, P, and Q). DAPI staining detects nuclei (gray; C, H, and M). (E, G, J, L, O, and Q) Electroporated cells are labeled with nuclear EGFP. (G, L, and Q) Insets show individual green channels. White lines highlight non–cell-autonomous NKX6.1 repression (I and J) and ectopic PAX7 expression (K and L). (K and L) Arrowheads demarcate the ventral most electroporated cell. Arrows indicate cell-autonomous inhibition of NKX6.1 expression (N and O) and ectopic PAX7 expression (P and Q) resulting from mHHIP1::CD4 expression. (N and O) Insets in represent embryos with dorsally restricted HHIP1::CD4 expression. Bars, 50 µm.