Fig. 1.
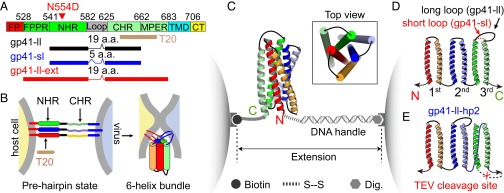
Sequences, conformations, and constructs of gp41 and the experimental setup. (A) Domain structure of HIV-1 gp41. The three truncated gp41 sequences used in this study, the sequence of T20, and T20-resistant mutation (N554D) are indicated. CHR, C-terminal heptad repeat; CT, cytoplasmic tail; FP, fusion peptide; FPPR, fusion peptide-proximal region; MPER, membrane-proximal external region; NHR, N-terminal heptad repeat; TMD, transmembrane domain. (B) Diagram showing gp41 folding and viral membrane fusion. In the prehairpin state, three gp41 monomers form a stable three-helix bundle in their NHRs with largely unfolded CHRs. We refer to the conformational transition from this prehairpin state to the six-bundle (6HB) state as gp41 folding, in contrast to de novo formation of the 6HB from free gp41 monomers as measured in previous ensemble experiments (18). (C) Experimental setup to pull a single gp41 complex using dual-trap optical tweezers. (D) Topology of the construct used to pull the third hairpin in the gp41 complex. Two constructs with different loop sizes were made, gp41-ll with a 19-aa loop and gp41-sl with a 5-aa loop. (E) Topology of the construct to specifically pull the second gp41 hairpin. The construct contained a cleavage site of the TEV protease (indicated by x). After TEV cleavage and purification, the C-terminal pulling site of gp41-ll-hp2 was generated.
