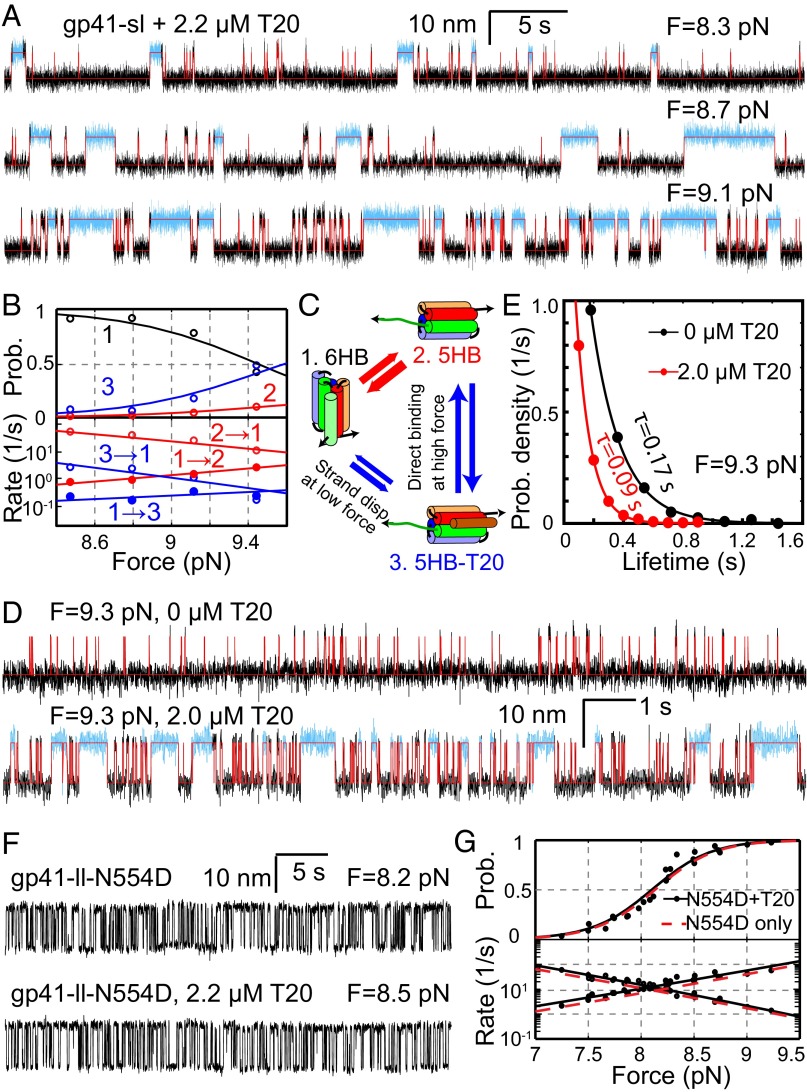
Fig. 5.
T20 inhibits folding of wild-type gp41, but not T20-resistant N554D mutant. (A) Extension-time trajectories of gp41-sl under different constant forces in the presence of 2.2 µM T20. The measured extensions were analyzed by the three-state HMM (red traces), revealing the 6HB state and the free and T20-bound 5HB states, with the T20-bound 5HB state highlighted in cyan. (B) Force-dependent state probabilities (Upper) and transition rates (Lower) of the gp41-sl complex in the presence of T20. (C) Diagram showing three-state transitions of gp41-sl with significant force-dependent and T20-induced 6HB unfolding and binding. (D) Extension-time trajectories of the same gp41-sl molecule under 9.3 pN force in the absence (Upper) or presence (Lower) of 2.0 µM T20. (E) Dwell-time probability density distributions of the 6HB state of gp41-sl (dots) and their single-exponential fits (lines) corresponding to traces in D. For better comparison, each measured distribution is normalized by its maximum. (F) Extension-time trajectories of gp41-ll-N554D with the indicated forces and T20 concentrations. (G) Force-dependent unfolding probabilities (Upper) and transition rates (Lower) of gp41-ll-N554D in the presence (black) and absence (red) of 2.2 µM T20. The measurements (black symbols) were well fit by a theoretical model (black lines). For clarity, only the best-fit line (red, Fig. 2C) is shown here for gp41-ll-N554D in the absence of T20.