Abstract
In vivo animal experiments are critical in the process of finding and developing new treatments for children with CNS tumors. Cerebral microdialysis, which enables researchers to measure drug concentrations in the brain or tumor tissue of unanesthetized mice, is a highly specialized procedure that provides valuable information that cannot be gained by using an in vitro system. When designing any in vivo animal study, 3 Rs principles (replacement, reduction, and refinement) must be considered to ensure that the highest standards of care are followed. As part of the refinement process, the objectives of this study were to collect behavioral monitoring data from mice undergoing cerebral microdialysis, to identify any behaviors predictive of significant pain or distress that could affect the animal's welfare, and to use these data to refine the existing monitoring checklist and schedule for its use by others performing this procedure. We developed a monitoring checklist for assessing wellbeing and distress of mice during cerebral microdialysis experiments. Comparison of 79 mice that underwent cerebral microdialysis experiments with a control group of 20 mice revealed that cerebral microdialysis and tethering of mice are well tolerated for as long as 24 h with only minor evidence of stress.
Abbreviation: MOCS, microdialysis observation checklist and schedule
Unacceptable cure rates and the adverse effects of current therapies highlight the crucial need to develop novel therapies for children with primary CNS malignancies. Despite the development of sophisticated in vitro techniques and ex vivo methodologies that aid in identifying new anticancer drugs for the treatment of CNS tumors, animal studies such as cerebral microdialysis provide important insights to enhance the translation of in vitro findings to in vivo studies and then into clinical trials in children. Cerebral microdialysis is a valuable tool for determining brain and tumor penetration of drugs in unanesthetized mice.2,16 During these studies, cannulated mice are tethered but able to move freely about the cage throughout the experiment. However, how to monitor these animals for pain and distress when they are treated with a chemotherapeutic agent and restrained with a tether for a prolonged time (for example, between 6 and 24 h) as part of the cerebral microdialysis procedure is unknown. In addition, due to the invasive nature of the cannula placement, persons unfamiliar with the microdialysis technique may raise concerns about the animal welfare and distress during the preparatory surgery and subsequent study. Furthermore, the engraftment of the xenograft in the brain and implantation of the cannula and head cap on the skull followed by cerebral microdialysis raises possible concerns regarding the welfare of the mice throughout the course of the experiment.
The critical role of laboratory animals in preclinical studies makes it imperative to balance ethical and animal welfare concerns with the need to perform complex studies in the pursuit of new cures. The optimal design for any animal study, including cerebral microdialysis, should consider the 3 Rs: replacement, reduction, and refinement.15
Cerebral microdialysis is an excellent example of the application of the reduction principle. Assessing the CNS penetration of drugs by using traditional pharmacokinetic studies generally involves sampling multiple mice per time point of interest, and the mice often must be euthanized to obtain sufficient sample and brain tissue volume. In contrast, cerebral microdialysis involves continuous collection of extracellular fluid samples and limited plasma sampling from individual mice. During these experiments, the disposition of a compound in the extracellular fluid and plasma over time can be determined in a single mouse, thus markedly reducing the number of mice required to determine whether a compound's pharmacokinetic properties merit its further development.Refinement aims to reduce adverse effects and improve the welfare of animals during a study and involves continual review of experimental procedures to minimize pain and suffering and improve welfare. As part of refinement, researchers must identify adverse effects and humane endpoints, monitor study animals appropriately to ensure that their health and welfare are maintained, assure that adverse effects are minimized and animals do not become moribund, and maintain detailed records on each animal involved. During the refinement process, the nature of some animal experiments may hamper assessment of the health and welfare of animals and the identification of adverse effects during the study, such as when animals are restrained or housed in specialized enclosures. For example, comprehensive, rapid, simple, and noninvasive animal monitoring techniques for use during cerebral microdialysis are needed. Therefore, the objectives of our current study were to collect behavioral assessment monitoring data from mice undergoing cerebral microdialysis to identify behaviors indicative of pain or distress that would affect animal welfare and to use these data to generate an appropriate observational checklist and schedule for assessing pain and distress during cerebral microdialysis in mice.
Materials/Methods
Animals.
Female CD1 nude mice (CD1 nu/nu; age, 3 to 4 wk) were purchased from Charles River Laboratories (Kingston, NY) and housed in groups of 5 in microisolation caging on 1/4-in. corncob bedding (Bed-O-Cob, Anderson Lab Bedding Products, Maumee, OH). Pelleted or ground autoclavable rodent diet (no. 5013, Purina LabDiet, Richmond, IN) and reverse-osmosis–purified water chlorinated to 8 ppm were provided ad libitum. Ground diet mixed with reverse-osmosis (RO) water was prepared as a soft diet for all mice. Lighting was maintained on a 12:12-h light: dark cycle, and the ambient conditions in the animal holding room throughout the study were 22 to 24 °C and 30% to 70% humidity. All microisolation caging materials, bedding, and feed were autoclaved prior to use. Cages were changed weekly in a class 2A biologic safety cabinet. All procedures were reviewed and approved by the St Jude Children's Research Hospital Animal Care and Use Committee. The animal care program is fully AAALAC-accredited.
Observational control animals.
Female CD1 nu/nu mice (n = 20; age, 6 to 8 wk) were weighed and anesthetized with isoflurane and oxygen. Once the mice were sedated, a small black plastic collar (CMA Microdialysis, Kista, Sweden) was securely placed around each animal's neck, and the mouse was returned to its home cage to acclimate for 1 h. The collar, used to connect the mouse to the tether and swivel system through which the microdialysis tubing was connected, allowed for free movement of the mouse without tangling the microdialysis tubing. Mice then were anesthetized again by using isoflurane in oxygen and each was placed in a clear microdialysis chamber (height, 15 in.; diameter, 10.5 in.; Instech Laboratories, Plymouth Meeting, PA). The chamber contained bedding and food from the animal's home cage, as well as water, soft diet, and hydration gel. All control animals (n = 20) were observed according to the 24-h observation schedule (see Observations Section following).
Experimental animals.
Between 6 and 8 wk of age, all experimental animals (n=79) monitored in this study underwent sterile survival surgery to implant microdialysis cannulas. The microdialysis cannula consists of a base of dental cement, a guide cannula, and a stylet. For tumor-bearing animals, tumor-type-specific cells (for example, ependymoma, choroid plexus carcinoma, glioma, medulloblastoma) labeled with luciferase were injected into the brain when the microdialysis cannula was implanted.5 Anesthesia was induced and maintained with ketamine (50 mg/kg; Hospira, Lake Forest, IL) and xylazine (10 mg/kg; VedCo, St Joseph, MO). The head of the mouse was fixed in position on a stereotactic apparatus (KOPF, Tujunga, CA). A small incision on the midline of the scalp was made, and the bregma was identified. A 0.7-mm hole was drilled in the skull, and the microdialysis guide cannula (BASi, West Lafayette, IN) was implanted into the cortex of the mouse by using the coordinates x = 1, y = 2, and z = –2.5 mm relative to bregma. The cannula was fixed to the skull by using cement (BASi, West Lafayette, IN; Figure 1A). Ibuprofen was given as an analgesic at a dosage of 40 mg/kg administered in drinking water for a minimum of 48 h after cannula implantation. Cerebral microdialysis in all nontumor-bearing mice was performed on days 3 to 5 after cannula implantation.
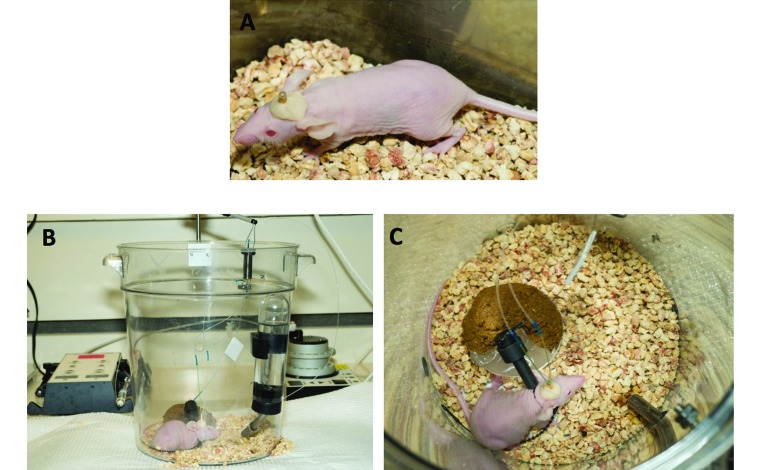
Figure 1.
Cerebral microdialysis in mice. (A) CD1 nu/nu mouse with cannula and dental cement head cap. (B) CD1 nu/nu mouse undergoing cerebral microdialysis; a syringe pump drives perfusate though tubing into the microdialysis probe inserted in the cannula placed in the mouse brain. Dialysate sample is collected through the outlet of the probe and outlet tubing inserted into a fraction collector. (C) CD1 nu/nu mouse attached to the swivel-tether apparatus; soft food, hydration gel, and water are present in the enclosure, along with bedding from original mouse cage.
Tumor-bearing mice were 8 to 12 wk old at the time of the microdialysis and monitoring experiments. Tumor engraftment was assessed weekly by bioluminescence imaging. Briefly, mice were anesthetized with isoflurane and oxygen and injected intraperitoneally with 200 μL d-luciferin (15 mg/mL; Caliper Life Science, Hopkinton, MA). Images of the head were collected 5 min after luciferin injection by using an in vivo imaging system (IVIS200, Xenogen, Caliper Life Science). Mice bearing brain tumors were assessed daily by the same observer, to ensure that guide cannulas were in place and there were no signs of infection at the implant site. In addition, until the microdialysis study (typically 2 to 4 wk after implantation), tumor-bearing mice were observed daily for any neurologic deficits associated with tumor growth (that is, head dome, circling, head tilt, weight loss, hunched posture). If any of these signs were present, the affected mouse was excluded from the microdialysis procedure and observation. Microdialysis experiments began once tumors reached sufficient size as determined by bioluminescence (approximately 108 photons per second, which corresponds to an average tumor volume of 30 to 35 mm3; typically 2 to 4 wk after implantation, depending on tumor type and growth characteristics).
On the day of cerebral microdialysis, each tumor-bearing or nontumor-bearing mouse was weighed and anesthetized with isoflurane in oxygen. Once anesthetized, a small black collar was placed around its neck and the mouse was returned to its cage to recover from anesthesia. After the mouse had acclimated to the collar for 1 h, it was anesthetized again for removal of the stylet from the cannula followed by insertion of a 1-mm microdialysis probe (BASi, West Lafayette, IN) into the cannula in place of the stylet. The mouse was placed in a clear cylindrical enclosure specifically designed for the procedure (described earlier), and a swivel–tether apparatus (CMA Microdialysis) was attached to the collar (Figure 1 B and C). Isotonic artificial cerebrospinal fluid (148 mM NaCl, 4 mM KCl, 0.8 mM MgCl2, 1.4 mM CaCl2, 1.2 mM Na2HPO4, 0.3 mM NaH2PO4) was perfused through small-diameter tubing (BASi) and the swivel connected to the inlet of the microdialysis probe by using a syringe pump (CMA102, CMA Microdialysis) at a flow rate of 0.5 µL/min.3 After a 1 h equilibration period, the antitumor drug was administered intravenously via a tail vein, intraperitoneally, or by oral gavage. Microdialysate samples were collected by using a fraction collector (CMA Microdialysis) through small-diameter tubing connected to the microdialysis probe outlet. Samples (dialysate and blood) were collected for various lengths of time (6 to 24 h), depending on the drug. In addition, 3 blood samples at pharmacokinetically determined optimal sampling time points were collected from the retroorbital plexus of each mouse, which was anesthetized briefly with 2% isoflurane in oxygen.
Compounds.
Mice used for cerebral microdialysis studies received both FDA-approved and investigational chemotherapeutic agents. These compounds included antifolates, nucleotide analogs, tyrosine kinase inhibitors, dual IGF1R–insulin receptor kinase inhibitors, polo-like kinase 1 inhibitors, and HMG–CoA reductase inhibitors. Each mouse received a single dose that was lower than the maximal tolerated dose either intravenously via a tail vein, intraperitoneally, or orally by gavage.
Observations.
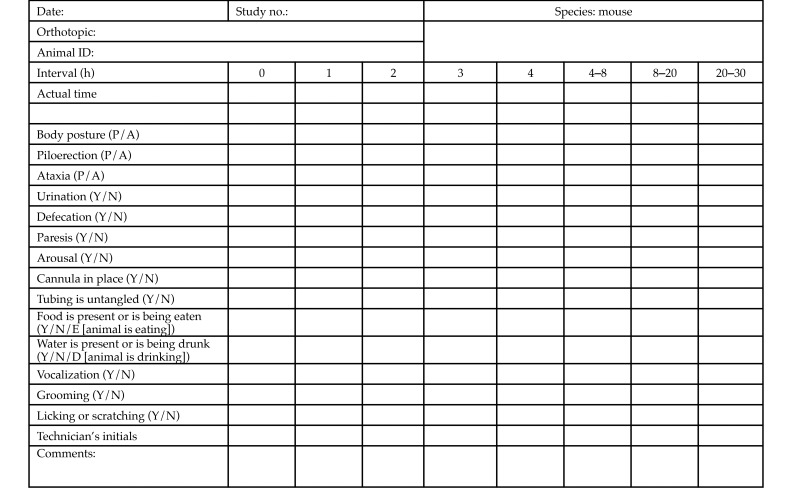
All observations were performed with intent for intervention as needed to maintain animal wellbeing. A Microdialysis Observational Checklist and Schedule (MOCS) was developed in conjunction with the principal investigator and his staff, the St. Jude Institutional Animal Care and Use Committee (IACUC), and the veterinary staff of the Animal Resources Center to assess some of the behaviors associated with pain and distress and drug toxicity during cerebral microdialysis. The parameters were taken from the Irwin Observation Test Battery and the primary screen of the SHIRPA protocol.8,14 The parameters used in these studies were limited to assessing the undisturbed behavior of mice in the enclosure where they were housed during the microdialysis procedure. In microdialysis experiments that lasted 6 to 8 h, mice were monitored for all 16 parameters at least once each hour for the duration of the experiment. In 10- to 12-h microdialysis experiments, mice were monitored hourly for the first 4 h and then at least once every 4 h thereafter. For 24-h microdialysis experiments, mice were monitored hourly for the first 4 h, once more over the next 4-h time period, and then twice over the next 16 h (with a maximal interval of 12 h between observations). Three technicians from the principal investigator's lab were trained extensively regarding the specific signs and symptoms to observe for each of the categories listed on the MOCS. Competency was achieved once a second technician validated the trainee's observations. Before a technician observed the mice independently, a second observer validated the first technician's observations. Observations were made at designated time points during all cerebral microdialysis experiments performed between August 2012 and August 2013. As shown in Figure 2, each mouse was specifically observed for the presence or absence of normal body posture, piloerection, ataxia, urination, defecation, paresis, arousal, placement of cannula, correct placement of tubing, presence of food and water in the microdialysis enclosure, eating, drinking, vocalization, grooming, and licking and scratching. Each observation period lasted 5 to 10 min. The time of each observation was recorded on the mouse-specific observation form (Figure 2), and all studies began between 0700 and 1200. For nighttime observations (1800–0600), the mice were kept in rooms equipped with red lights, which are routinely used in the facility during the animals’ 12-h dark phase.
Figure 2.
Microdialysis observation checklist and schedule for a 24-h microdialysis experiment. A, absent; N, no; P, present; Y, yes.
Statistics.
Statistical analyses were performed by using GraphPad Prism Software, (GraphPad Software, San Diego, CA) Student unpaired t tests, a Kaplan–Meier survival curve, and Fisher exact tests.
Results
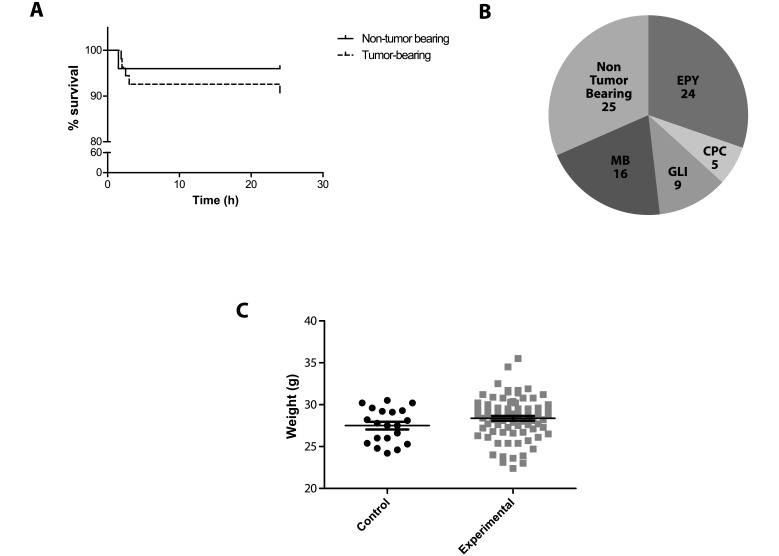
Between August 2012 and August 2013, we observed a total of 79 mice that underwent a cerebral microdialysis procedure. All were adult female CD1 nude mice, and 25 were nontumor-bearing mice. Of the 79 experimental mice, 6 died spontaneously during the cerebral microdialysis studies, yielding an overall mortality rate of 7.6%. Survival did not differ between nontumor-bearing (n = 25) and tumor-bearing mice (n = 54; Figure 3 A). One tumor-bearing mouse died while being observed within the first 2 h of the study, another 3 tumor-bearing mice died while being observed during hours 2 through 4 of the study, and one mouse was found dead 23 h after the microdialysis experiment began. A single nontumor-bearing mouse died 1.5 h after the experiment began. We did not observe any adverse signs in these mice prior to death, and the causes of death remain unknown.
Figure 3.
Characteristics of mice observed. (A) Survival curves for the 25 nontumor-bearing experimental mice and 54 tumor-bearing experimental mice observed during cerebral microdialysis showing that all but 1 death occurred during the first 4 h of observation (P = 0.43). (B) Number of mice observed for each tumor model. A total of 54 CNS tumor-bearing mice were included in the experimental group; mice were implanted with ependymoma (EPY), choroid plexus carcinoma (CPC), pediatric glioma (GLI), or medulloblastoma (MB) tumor cells. (C) Comparison of body weight between control and experimental mice.
Once a cannula or microdialysis probe is displaced, the mouse must be removed from the study because the microdialysate samples needed to accomplish the goals of the study cannot be collected. Nine mice had to be removed from the study prior to completion due to problems with cannula or probe placement (n = 5) or lack of perfusate flow (n = 4); however, observations of these animals that were completed prior to study removal were included in the analyses.
For nontumor-bearing mice, microdialysate samples were collected from the extracellular fluid of the normal brain cortex; the remaining 54 mice harbored pediatric brain tumors, and samples were collected from the tumor extracellular fluid. Of these 54 mice, 24 were implanted with ependymoma tumor cells, 5 were implanted with a choroid plexus carcinoma cell line, and 9 were implanted with pediatric glioma tumors; the remaining 16 mice were implanted with medulloblastoma cells (Figure 2 B). Tumor-bearing mice underwent bioluminescence imaging, which yielded an average bioluminescence signal of 3.13 × 108 photons per second (corresponding to a tumor volume of approximately 36 mm3), 24 h prior to cerebral microdialysis. These animals showed no sign of neurologic impairment as a result of tumor burden at the time of the microdialysis experiment. We did not observe any noteworthy difference between nontumor-bearing and tumor-bearing mice in regard to any observational measures made during microdialysis studies; therefore, these 79 mice will collectively be referred to as the experimental group. On the day of monitoring, the control mice had an average weight of 27.5 ± 2.0 g compared with 28.4 ± 2.5 g for the experimental group on day of cerebral microdialysis (P > 0.05; Figure 3 C).
All mice monitored during this study were observed for both normal and abnormal behavior and events by using the appropriate MOCS. During the microdialysis procedure observation periods, the percentage of mice eating was lower for the experimental compared with the control mice (72% compared to 95%, p=0.04), but drinking was similar between groups (26.6% of experimental mice vs. 40% of control mice, p>0.05). Corresponding to these data, the percentage of experimental mice that defecated was lower for experimental than control mice (79.9% compared to 100%, p=0.04), whereas the percentage of mice that were observed urinating was similar between groups (46.8% of experimental mice and 40% of control mice, p>0.05). We also noted a decrease in the percentage of experimental mice that groomed themselves when compared with control mice (50.6% versus 80%, p=0.02). We did not observe any piloerection events in either control or experimental mice, suggesting that the autonomic functions of the mice were not compromised.13 At all of the observation time points, neurologic symptoms were monitored by observing for the presence of paresis, arousal, ataxia, and abnormal body posture. No occurrence of paresis or ataxia was observed in either control or experimental mice. Although no episodes of abnormal posture were observed in the control mice, 5 such episodes occurred in 2 mice among a total of 8032 observations (<0.1%). Interestingly, the same 2 mice both displayed clinical signs of a hypertonic response, as evidenced by extension of one of the hindlegs. In one mouse, this response was observed 19 and 24 h after the microdialysis experiment began; this mouse had received an FDA-approved dual IGF1R–insulin receptor kinase inhibitor. The second mouse, which had been dosed with an FDA-approved antifolate drug, showed this response at 4, 5, and 8 h after the experiment began. Notably, in both mice, these responses were temporary, did not cause any adverse effects, and did not prevent the mice from completing the microdialysis experiment as planned.
To ensure that the time points selected for monitoring did not exclude behavioral changes, more frequent prolonged monitoring was performed in 4 of the 79 experimental animals. No a priori information was available to guide the selection of time points for this prolonged monitoring, which had to be done in a subset of animals due to limited personnel and resources; the concern for animal welfare had to be balanced with practicality. Therefore, these 4 animals were observed every 2 h throughout the entire 24-h cerebral microdialysis experiments. Because mice are nocturnal animals, observing on this schedule ruled out the possibility of missed adverse effects, pain, or distress that were occurring during the night, when the mice were most active. Even when monitoring occurred every 2 h, no adverse effects on the wellbeing of the mice were noted, demonstrating that the lack of adverse and behavioral effects observed from cerebral microdialysis was not due to insufficient monitoring time points.
Discussion
Cerebral microdialysis is a valuable technique that is used in cancer research to determine the disposition of anticancer agents in the brains and tumors of mice. Because of the complex nature of these experiments, we aimed to identify behaviors predictive of significant pain or distress that could affect the animal's wellbeing and the scientific endpoint of the experiment. This study was done as part of the refinement process involved in maintaining the highest standards of care for the animals involved in these experiments. Because when an animal is in pain and whether the pain is severe enough to threaten the animal's welfare are not always obvious, behavioral monitoring is a valuable tool that can be used in the refinement process.6 Behavioral monitoring is a practical, noninvasive way to monitor an animal's physical and behavioral parameters during experimental procedures to ensure that its welfare is not compromised.12 In the current study, we used a monitoring checklist and schedule that was based on the Irwin Observational Test Battery.8
The nature of cerebral microdialysis, which involves the tethering of cannulated mice for a prolonged period to obtain adequate sample volumes, poses a challenge to those assessing the stress level and welfare of the experimental mice, especially for observers or staff unfamiliar with the technique. In this study, we monitored 99 CD1 nude mice, 20 mice under control conditions and 79 mice undergoing restraint during cerebral microdialysis, to determine whether any single behavior or combination of behaviors was indicative of pain or distress and whether any of these observations had an effect on animal welfare. Our results demonstrate that during cerebral microdialysis experiments, mice do not demonstrate characteristics consistent with significant pain or distress that would suggest their wellbeing was compromised (that is, the mice maintain good physical health and are in the absence of undue stress7,19).
In our experience mice tolerate the collar–tether system well. However, the use of a collar with a swivel and tether restraint system often raises many concerns from those unfamiliar with the cerebral microdialysis technique. One of the most common concerns expressed by IACUC reviewers who are unfamiliar with these types of studies is that the mouse will entangle itself in this device. Notably, none of the 79 experimental animals in our study became entangled at any point. Another common concern is that one or both of a mouse's front legs will become trapped in the collar, thus preventing the animal from having free range of motion for the remainder of the experiment. Again, this problem did not arise during our study. However, we do recommend that the experimenter is trained regarding placing the collars on the mice and that the collar is adjusted during the 1-h acclimation period if it appears to be too tight or too loose. The final common issue raised by IACUC reviewers is that the tubing threaded through the swivel to the probe will tangle and restrict the movement of the animal. However, when the tubing, swivel, and tether are connected correctly, the tubing does not tangle and therefore does not present an animal welfare concern. In our 79 microdialysis experiments, we did not have any issues with the tangling of tubing.
Because of problems with cannula or probe placement, 5 mice were removed from the study before completion of microdialysis. Specifically, the cement that stabilizes the probe in the brain did not hold for the duration of the experiment, causing displacement of the cannula and probe. To decrease the frequency of this issue, we purchased an alternative brand of dental cement, the use of which has eliminated this problem.
Grooming events were monitored in both control and experimental mice according to the semiquantitative SHIRPA protocol used for high-throughput assessment of mouse phenotypes.14 We observed less grooming behavior in experimental mice than in control mice. Grooming is an important behavior that is not only useful for hygiene but also may serve other functions, such as social interaction, de-arousal, and reducing stress.17 Several studies have examined the grooming habits of rats and mice in response to stress, both acute and chronic.9,11,18 In some strains of mice (for example, C57BL/6, CBA and CC57BR), chronic stress significantly suppresses grooming activity, whereas the effect of acute stress on grooming patterns varies depending on the stressor (that is, restraint stress causes a significant increase in grooming).10,18 In all of these studies, detailed analyses of grooming behavior patterns (that is, length of bout, direction of grooming, time between bouts) were used to determine the effects of stress on grooming, and the results varied among strains of mice, species of animals, and stressors present. Although our results demonstrate a decrease in the percentage of mice grooming in our experimental group compared with controls, whether this result was due to increased stress cannot be determined, given the conflicting studies relating grooming and stress. It is important to note that in no mice did we observe a decrease in the overall wellbeing during microdialysis. In light of the data from our MOCS, future observational checklists may need to include additional grooming analysis (such as length of bout, average time between bouts, direction of grooming) to accurately examine an animal's level of pain and distress.
The current study has several limitations. We used our MOCS to evaluate single strain, sex, and source of animals undergoing a single procedure. In this study, we observed a large number of mice to evaluate the presence or absence of stress-related behavioral changes during cerebral microdialysis; whether similar behavioral observation would be beneficial for other species undergoing similar experiments is unknown. In addition, although many researchers use cerebral microdialysis in rats for pharmacokinetic studies, reports describing the stress and general welfare of rats during these studies are scarce. The procedure followed and experimental set-up used for cerebral microdialysis in rats is very similar to methods used in the current study. Therefore, the implementation of this observational checklist in rats likely is feasible, but preliminary studies validating the use of this checklist should be performed prior to its implementation. In addition, further evaluations will be necessary to determine the applicability of observational monitoring in other species undergoing similar experiments.
The MOCS used in the current study provides evidence that the cerebral microdialysis procedure does not significantly affect the wellbeing of mice. For other experimental procedures, it may be necessary to use more extensive behavioral observations to ensure the wellbeing of the animals. Currently, some researchers rely on automated, behavioral phenotyping instruments to examine behavioral alterations over long periods of time with minimal human intervention.1,4 Although these instruments are beneficial for high-throughput behavioral analysis, many evaluate only a small number of behaviors and rely primarily on video analysis, which would preclude any interventions that may become necessary.4 Therefore the use of automated, behavioral monitoring equipment should be considered carefully when examining new CNS compounds or novel mouse models representing a particular CNS disease.
In conclusion, the overall survival rate for the 79 mice observed between August 2012 and August 2013 was 92.4%, and none of the animals exhibited signs of severe pain or distress as a consequence of the microdialysis procedure. The data collected using the observation checklist is in accordance with the subjective findings from our lab over the past 15 y. The behavioral monitoring data presented here suggests that although cerebral microdialysis and tethering of animals may cause minor distress in the mice (as evidenced by the intergroup differences in eating, defecating and grooming), overt distress is not apparent, and the procedure is well tolerated for as long as 24 h.
Acknowledgments
We acknowledge the support of Cancer Center Support CORE Grant P30 CA 21765 from the National Cancer Institute, The Collaborative Ependymoma Research Network (CERN), The V Foundation, and the American Lebanese Syrian Associated Charities. We thank the members of St. Jude Children's Research Hospital Animal Resource Center, Dr Amy Funk, and St Jude Children's Research Hospital Biomedical Communications.
References
- 1.Balci F, Oakeshott S, Shamy JL, El-Khodor BF, Filippov I, Mushlin R, Port R, Connor D, Paintdakhi A, Menalled L, Ramboz S, Howland D, Kwak S, Brunner D. 2013. High-throughput automated phenotyping of 2 genetic mouse models of Huntington's disease. PLoS Curr 11:5 doi: 10.1371/currents.hd.124aa0d16753f88215776fba102ceb29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin RK, Hochberg FH, Fox E, Bungay PM, Elmquist WF, Stewart CF, Gallo JM, Collins JM, Pelletier RP, de Groot JF, Hickner RC, Cavus I, Grossman SA, Colvin OM. 2004. Review of microdialysis in brain tumors, from concept to application: 1st annual Carolyn Frye-Halloran symposium. Neuro-oncology 6:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benveniste H, Hüttemeir PC. 1990. Microdialysis–theory and application. Prog Neurobiol 35:195–215. [DOI] [PubMed] [Google Scholar]
- 4.Brodkin J, Frank D, Grippo R, Hausfater M, Gulinello M, Achterholt N, Gutzen C. 2014. Validation and implementation of a novel high-throughput behavioral phenotyping instrument for mice. J Neurosci Methods 224:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carcaboso AM, Elmeliegy MA, Shen J, Juel SJ, Zhang ZM, Calabrese C, Tracey L, Waters CM, Stewart CF. 2010. Tyrosine kinase inhibitor gefitinib enhances topotecan penetration of gliomas. Cancer Res 70:4499–4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carstens E, Moberg GP. 2000. Recognizing pain and distress in laboratory animals. ILAR J 41:62–71. [DOI] [PubMed] [Google Scholar]
- 7.Gebhart GF, Basbaum AI, Bird SJ, Flecknell P, Goodly L, Karas AZ, Kelley ST, Lacher J, Mason G, Sneddon LU, Soriana SG. 2009. Recognition and Alleviation of Pain in Laboratory Animals Pittsburg (PA): National Academies Press. [Google Scholar]
- 8.Irwin S. 1968. Comprehensive observational assessment: Ia. A systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharmacology 13:222–257. [DOI] [PubMed] [Google Scholar]
- 9.Kalueff AV, Tuohimaa P. 2004. Grooming analysis algorithm for neurobehavioural stress research. Brain Res Brain Res Protoc 13:151–158. [DOI] [PubMed] [Google Scholar]
- 10.Kulikov AV, Tikhonova MA, Kulikova EA, Kulikov VA, Popova NK. 2010. [Effect of genotype and emotional stress on hygienic grooming in inbred mice] Zh Vyssh Nerv Deiat Im I P Pavlova 60:632–637. [In Russian.] [PubMed] [Google Scholar]
- 11.Mineur YS, Prasol DJ, Belzung C, Crusio WE. 2003. Agonistic behavior and unpredictable chronic mild stress in mice. Behav Genet 33:513–519. [DOI] [PubMed] [Google Scholar]
- 12.Morton DB, Griffiths PH. 1985. Guidelines on the recognition of pain, distress and discomfort in experimental animals and an hypothesis for assessment. Vet Rec 116:431–436. [DOI] [PubMed] [Google Scholar]
- 13.Rogers DC, Fisher EM, Brown SD, Peters J, Hunter AJ, Martin JE. 1997. Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm Genome 8:711–713. [DOI] [PubMed] [Google Scholar]
- 14.Rogers DC, Peters J, Martin JE, Ball S, Nicholson SJ, Witherden AS, Hafezparast M, Latcham J, Robinson TL, Quilter CA, Fisher EM. 2001. SHIRPA, a protocol for behavioral assessment: validation for longitudinal study of neurological dysfunction in mice. Neurosci Lett 306:89–92. [DOI] [PubMed] [Google Scholar]
- 15.Russell WMS, Burch RL. 1959. The principles of humane experimental technique. London (UK): Methuen and Company. [Google Scholar]
- 16.Shen J, Fraga C, Calabrese C, McCarville MB, Schaiquevich P, Stewart CF. 2008. A modified surgical procedure for microdialysis probe implantation in the lateral ventricle of a FVB mouse. J Pharm Sci 97:5013–5023. [DOI] [PubMed] [Google Scholar]
- 17.Spruijt BM, van Hooff JA, Gispen WH. 1992. Ethology and neurobiology of grooming behavior.Physiol Rev 72:825–852. [DOI] [PubMed] [Google Scholar]
- 18.van Erp AM, Kruk MR, Meelis W, Willekens-Bramer DC. 1994. Effect of environmental stressors on time course, variability, and form of self-grooming in the rat: handling, social contact, defeat, novelty, restraint, and fur moistening. Behav Brain Res 65:47–55. [DOI] [PubMed] [Google Scholar]
- 19.Young RJ. 2003. Environmental enrichment for captive animals. Oxford (UK): Blackwell Science. [Google Scholar]