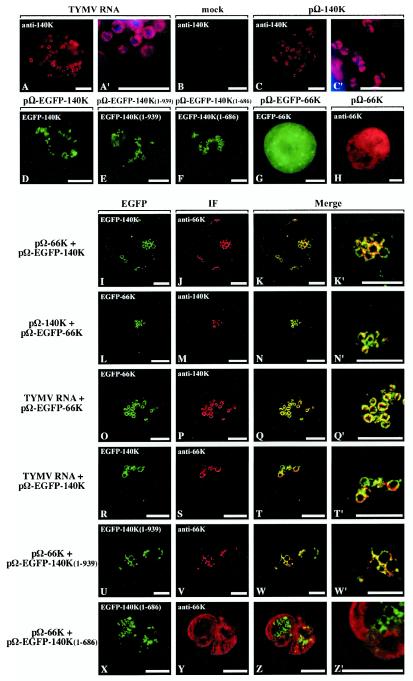
FIG.2.
Colocalization of 66K and 140K-derived proteins within Arabidopsis cells. Arabidopsis protoplasts were transfected with viral RNA (A and A′); with water (B); with expression plasmid pΩ-140K (C and C′), pΩ-EGFP-140K (D), pΩ-EGFP-140K(1-939) (E), pΩ-EGFP-140K(1- 686) (F), pΩ-EGFP-66K (G), or pΩ-66K (H); with plasmid pΩ-66K together with pΩ-EGFP-140K (I to K′); with plasmid pΩ-140K together with pΩ-EGFP-66K (L to N′); with plasmid pΩ-EGFP-66K together with TYMV RNA (O to Q′); with plasmid pΩ-EGFP-140K together with TYMV RNA (R to T′); with plasmid pΩ-EGFP-140K(1-939) together with pΩ-66K (U to W′); or with plasmid pΩ-EGFP-140K(1- 686) together with pΩ-66K (X to Z′). The transfected protoplasts were collected at 24 h posttransfection (A to C′ and H to Z′) or 47 h posttransfection (D to G). The cells either were processed for indirect immunofluorescence labeling with anti-140K antiserum (A to C′ and L to Q′) or anti-66K antiserum (H to K′ and R to Z′) followed by secondary antibodies coupled to Alexa-fluor 594 or were observed for GFP fluorescence (D to G). Single protoplasts were observed either by epifluorescence microscopy (A to H) or by CLSM with sequential acquisition of EGFP fluorescence (green) (I, L, O, R, U, and X) and Alexa-fluor 594 fluorescence (red) (J, M, P, S, V, and Y). The merged images in panels K, N, Q, T, W, and Z represent digital superimpositions of red and green signals in which areas of fluorescence colocalization appear yellow. Scale bars, 10 μm. Panels A′, C′, K′, N′, Q′, T′, W′, and Z′ correspond to enlargements of panels A, C, K, N, Q, T, W, and Z, respectively. In order to visualize the location of chloroplasts, DAPI staining (blue) of chloroplast DNA was acquired and superimposed onto the fluorescence signal of the viral protein (red) in panels A′ and C′.