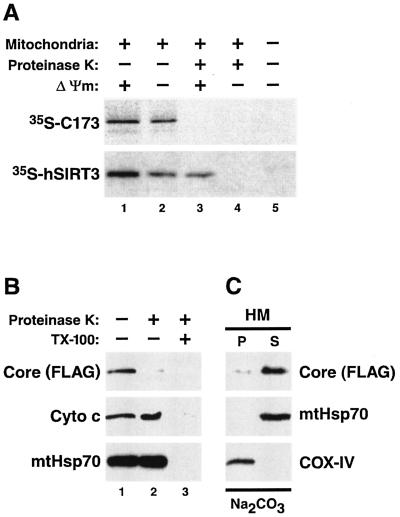
FIG. 5.
Nonintegral attachment of core protein to the mitochondrial outer membrane. (A) In vitro import assays using isolated mitochondria and in vitro-synthesized radiolabeled core protein (173 amino acids) or human SIRT3 (hSIRT3) were analyzed by autoradiography. The core protein was not imported into mitochondria and was accessible to the proteolytic activity of proteinase K (top, lane 3). SIRT3 was partially protected from digestion by proteinase K because of active mitochondrial import (bottom, lane 3). Accordingly, inhibitors of the mitochondrial membrane potential (Δψm) reduced the signal for SIRT3 (bottom, lane 2) but had no effect on the core protein (top, lane 2). SIRT3 was completely digested by proteinase K when active mitochondrial import was inhibited (bottom, lane 4). (B) Proteinase K digestion of mitochondria isolated from core protein-expressing Jurkat Tet-Off cells in the presence or absence of detergent (TX-100). A Western blot analysis showed that while the FLAG-tagged core protein was readily digested by proteinase K in the absence of TX-100 (lane 2), proteins located in the intermembrane space, such as cytochrome c (Cyto c), and mtHsp70, which is localized in the mitochondrial matrix, were protected. (C) Sodium carbonate extracts of HM fractions isolated from core protein-expressing Jurkat Tet-Off cells were analyzed for the distribution of the FLAG-tagged core protein, mtHsp70, which in the matrix is peripherally attached to the inner mitochondrial membrane, and COX-IV, a polytopic inner mitochondrial membrane protein.