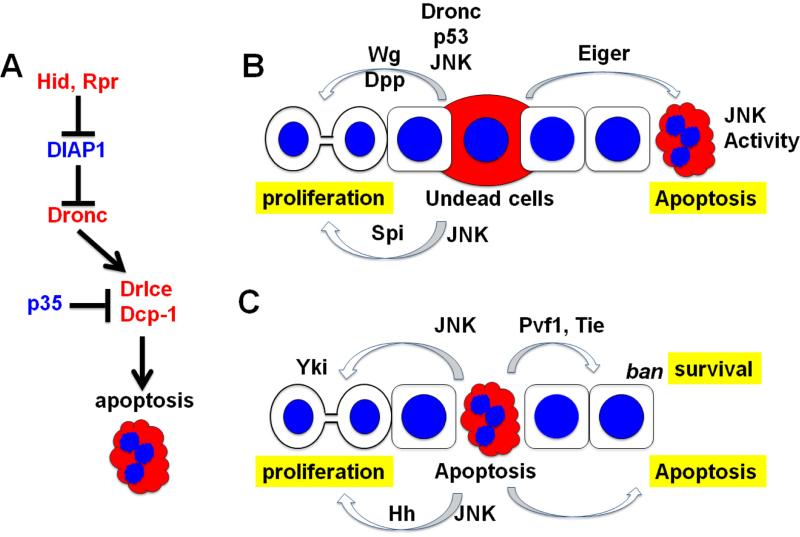
Figure 1.
(A) Drosophila cell death pathway. Only the proteins discussed in this review are shown. Pro-apoptotic proteins are in red and anti-apoptotic proteins are in blue. Initiator caspase Dronc cleave to activate effector caspases Drice and Dcp-1. Their activity is kept in check by DIAP1. In response to apoptotic stimuli, Hid and Rpr overcome the effect of DIAP1 to induce apoptosis. p35 does not interfere with caspase cleavage but inhibits their activity. Dronc is refractory to inhibition by p35. (B) Undead cell-induced proliferation (left) and apoptosis (right). Co-expression of p35 with pro-apoptotic proteins generates undead cells that have initiated apoptosis but cannot complete it. These induce non-autonomous proliferation through Wg, Dpp and Spi, and apoptosis through JNK activation. Wg induction by undead cells requires Dronc, JNK and p53 in undead cells. Spi induction by undead cells requires JNK in undead cells. JNK activation to induce non-autonomous apoptosis requires eiger in undead cells. (C) Genuine apoptosis-induced proliferation (left) and survival/apoptotic signaling (right). The proliferative response in the wing disc is mediated by JNK activity in the apoptotic cells that activates Yki in the nearby cells. The proliferative response in the differentiating regions of the eye disc is mediated by Hh. Survival signaling occurs by activation of ban miRNA in the protected cells, which is dependent on effector caspase activity, Tie and Pvf1. Apoptotic cells also induce non-autonomous apoptosis but the molecular basis for this response remains to be understood.