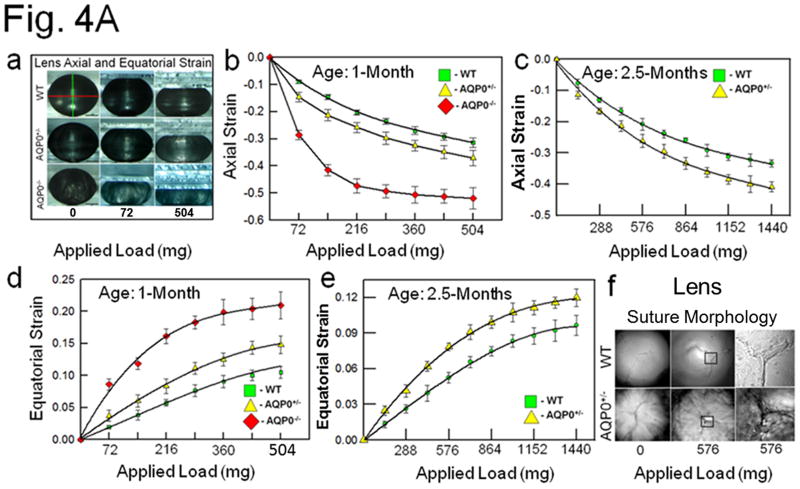
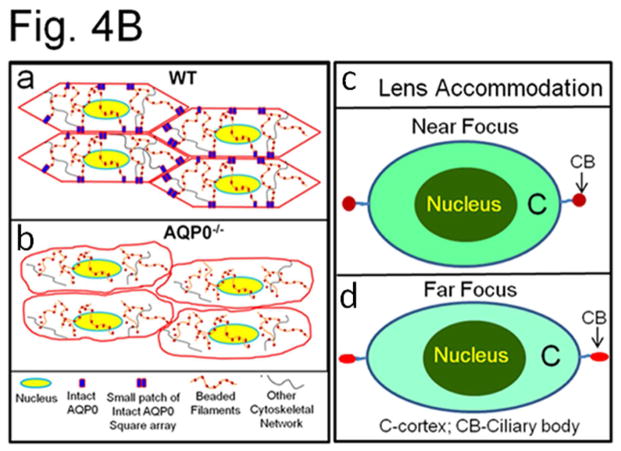
Fig. 4.
(A) (a) Compression stress test. Images of mouse lenses compressed by coverslips. Red line on WT - equatorial plane; green line - axial plane. Axial strain-load curves of: (b) 1-month-old WT, AQP0+/− and AQP0−/− mice and (c) 2.5 month-old lenses of WT and AQP0+/− mice. Equatorial strain-load curves of: (d) 1-month old WT, AQP0+/− and AQP0−/− mice and (e) 2.5 month-old lenses of WT and AQP0+/− mice. (f) Confocal imaging of lens suture morphology of WT and AQP0+/− mouse without (0 mg) or with (576 mg) coverslip squeezing. Small square areas denoted in the lenses are magnified to the right to show splitted suture area in AQP0+/− (bottom) and intact suture in WT (top). B. (a) Schematic model illustrating the role of AQP0 in lens biomechanics. Packed closer, outer cortical fiber cells of WT show characteristic hexagonal architecture with least extracellular space. Cytoskeletal proteins are tethered to AQP0 at the membrane. (b) Loss of fiber cell AQP0 causes loss of cellular architecture possibly due to loss of fiber to fiber adhesion and interaction with cytoskeletal networks. (c,d). Schematic of the possible mechanism of mammalian lens accommodation. While focusing near objects, ciliary muscles contract, the suspensory ligaments relax and the lens appears round (4Bc). While focusing far objects, the opposite events happen (4Bd). Note, the shape of the lens nucleus does not change. AQP0 may facilitate lens accommodation by establishing appropriate spatial biomechanics at the cortex and nucleus.