Abstract
The aim is to evaluate how language experience (Chinese, English) shapes processing of pitch contours as reflected in the amplitude of cortical pitch response components. Responses were elicited from three dynamic, curvilinear, nonspeech stimuli varying in pitch direction and location of peak acceleration: Mandarin lexical Tone2 (rising) and Tone4 (falling); and a flipped variant of Tone2, Tone2′ (nonnative). At temporal sites (T7/T8), Chinese Na-Pb response amplitude to Tones 2 & 4 was greater than English in the right hemisphere only; a rightward asymmetry for Tones 2 & 4 was restricted to the Chinese group. In common to both Fz-to-linked T7/T8 and T7/T8 electrode sites, the stimulus pattern (Tones 2 & 4 > Tone2′) was found in the Chinese group only. As reflected by Pb-Nb at Fz, Chinese amplitude was larger than English in response to Tones 2 & 4; and Tones 2 & 4 were larger than Tone2′; whereas for English, Tone2 was larger than Tone2′ and Tone4. At frontal electrode sites (F3/F4), regardless of component or hemisphere, Chinese responses were larger in amplitude than English across stimuli. For either group, responses to Tones 2 & 4 were larger than Tone2′. No hemispheric asymmetry was observed at the frontal electrode sites. These findings highlight that cortical pitch response components are differentially modulated by experience-dependent, temporally distinct but functionally overlapping weighting of sensory and extrasensory effects on pitch processing of lexical tones in the right temporal lobe and, more broadly, are consistent with a distributed hierarchical predictive coding process.
Keywords: pitch acceleration, pitch direction, functional asymmetry, tone language, Mandarin Chinese
1. Introduction
Pitch processing is shaped by one’s experience with language and music at the level of the auditory brainstem as well as the cerebral cortex (Patel, 2008; Alho et al., 2012; Itoh et al., 2012; Koelsch, 2012; Krishnan et al., 2012). In tone languages, the primary auditory correlate of lexical tone is based on variations in pitch. As measured by the early, preattentive mismatch negativity (MMN), Mandarin tones, relative to consonants, are lateralized to the right hemisphere (RH) (Luo et al., 2006). Using sinusoidal tones, MMN has been shown to be comprised of temporally-distinct auditory and cognitive mechanisms of frequency change detection in auditory cortex (Maess et al., 2007). But MMN itself is not a pitch-specific response.
Tone languages give us a physiologic window to evaluate how neural representations of linguistic pitch emerge during early sensory processing. We have yet to achieve a precise characterization of neural representation of specific attributes of dynamic pitch contours. Given that parallel processing of neurophysiological indicators of psycholinguistic information occurs with near-simultaneity in the first 200–250 ms, (Pulvermuller et al., 2009), an early cortical pitch-specific response (CPR) is necessary to tease apart sensory and extrasensory influences on pitch.
Most of the previous studies measured cortical responses that were prominently obligatory responses to sound onset and not pitch-specific (Gutschalk et al., 2004; Lutkenhoner et al., 2006; Yrttiaho et al., 2008). Pitch stimuli were steady-state, non-occurring in natural speech. Only the onset component (N100) was measured, a response to the onset of sound energy, and not exclusively to pitch. The CPR, on the other hand, is characterized by multiple, transient components that index different temporal attributes of dynamic pitch contours. To disentangle the pitch-specific from the obligatory onset response, a novel stimulus paradigm was constructed with two segments: an initial segment of noise with no pitch to evoke the onset components only, followed by a pitch-eliciting segment of iterated rippled noise (IRN) (Krumbholz et al., 2003). Adapting this stimulus paradigm, the magnitude of CPR transient components elicited by dynamic pitch homologs of Mandarin Tone 2 (T2, rising), were larger in Chinese than English listeners (Krishnan et al., 2015). By employing dynamic, curvilinear pitch stimuli representing both native and nonnative contours, we have an opportunity to tease apart sensory and extrasensory influences on experience-dependent pitch processing.
Herein we examine a set of three dynamic, curvilinear, nonspeech pitch stimuli that differ in pitch direction and location of peak acceleration. Two are homologous to Mandarin T2 and Tone 4 (T4, falling); the third is a flipped variant of T2 (T2′). We evaluate the effects of experience-dependent (Chinese, English) effects on latency and amplitude of CPR components. Stimulus comparisons allow us to assess acoustic effects of location of peak acceleration and pitch direction. Stimulus comparisons at frontal and temporal electrode sites allow us to assess hemispheric asymmetry. We hypothesize that at the right temporal site, the pattern of changes in the CPR components reflect temporally distinct, differential weighting of sensory and extrasensory effects depending on language experience.
2. Materials and methods
Participants
Fourteen native speakers of Mandarin Chinese (7 male, 7 female) and English (10 male, 4 female) were recruited from the Purdue University student body to participate in the experiment. All exhibited normal hearing sensitivity at audiometric frequencies between 500 and 4000 Hz and reported no previous history of neurological or psychiatric illnesses. They were closely matched in age (Chinese: 24.14 ± 3.28 years; English: 22.36 ± 1.08), years of formal education (Chinese: 16.86 ± 2.11 years; English: 16.18 ± 1.44), and were strongly right handed (Chinese: 92.2± 11.8 %; English: 95.8 ± 9.2 %) as measured by the laterality index of the Edinburgh Handedness Inventory (Oldfield, 1971). All Chinese participants were born and raised in mainland China. None had received formal instruction in English before the age of nine (12.07 ± 1.94 years). As determined by a music history questionnaire (Wong & Perrachione, 2007), all Chinese and English participants had less than two years of musical training (Chinese, 0.64 ± 0.82 years; English, 0.71 ± 0.83) on any combination of instruments. No participant had any training within the past five years. Each participant was paid and gave informed consent in conformity with the 2013 World Medical Association Declaration of Helsinki and in compliance with an experimental protocol approved by the Institutional Review Board of Purdue University.
Stimuli
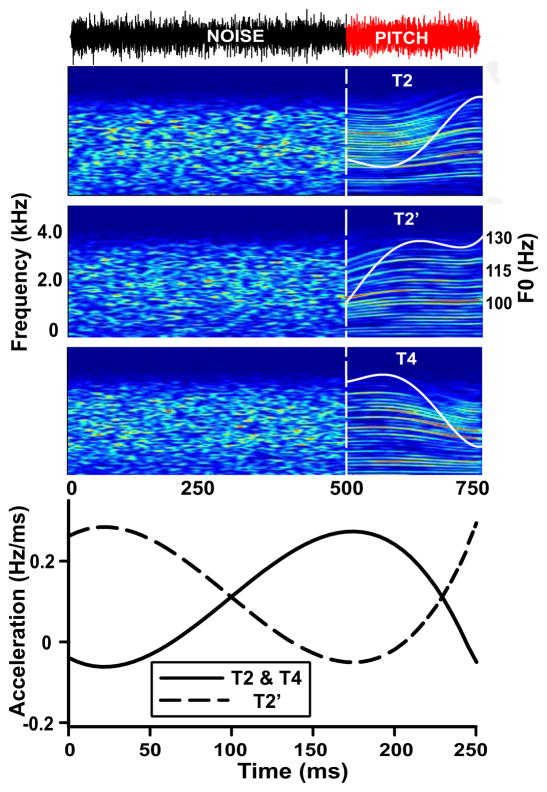
Three nonspeech stimuli were constructed to investigate CPR responses to curvilinear, time-varying pitch that differed in changes of direction (Fig. 1, top panel, right) and rates of acceleration (Fig. 1, bottom panel). Two of the stimuli represented lexical tones of Mandarin, modeled after productions of citation forms on isolated monosyllables of Tone 2 (T2, rising) and Tone 4 (T4, falling) (Howie, 1976; Moore & Jongman, 1997; Xu, 1997). T2 and T4 shared an average F0 of 111 Hz and pitch acceleration rate trajectory that reached its peak at 70% (175.69 ms) of total duration. They differed in the direction of pitch during those portions characterized by larger changes in F0 that are known to contribute importantly to tonal recognition (Whalen & Xu, 1992). The third stimulus (T2′, rising) does not exist in citation form in the Mandarin tonal space. Though T2′ represented a flipped variant of T2, it shared F0 onset/offset (103/131 Hz) in common with T2 as well as direction of pitch change (rising); its average F0 was 123 Hz. Despite differences in pitch direction, T2 and T4 shared the same acceleration trajectory throughout their duration. Both were 180° out of phase with T2′. T2 and T4 reached a late peak of acceleration at 70% of duration; T2′, in contrast, an early peak at about 10% (24.42 ms) of duration. Regardless of its location, the acceleration peak was constant across all three stimuli (0.28 Hz/ms). Duration was fixed at 250 ms across stimuli.
Figure 1.
(Top) Waveform (T2) and spectrograms of each of the three stimulus conditions (T2, T2′, T4) illustrate the experimental paradigm used to acquire cortical responses. The waveform shows robust periodicity within the pitch segment for T2. The vertical white, dashed line at 500 ms demarcates the transition from the initial noise segment to the final pitch segment. CPRs were extracted from evoked responses beginning with pitch onset. F0 contours (white) are superimposed on their respective pitch segments. T2 (rising) and T4 (falling), respectively, represent exemplary citation forms of Mandarin Tone 2 and Tone 4. T2′ (flipped variant of T2) exhibits a rising pitch contour similar in pitch direction to T2. Yet T2′ does not occur in the Mandarin tonal space Across stimuli, spectrograms show clear resolution of dynamic, rising or falling spectral bands corresponding to the harmonics of the fundamental frequency. (Bottom) In addition to changes in pitch direction, pitch contours are differentiated phonetically by changes in the location of peak acceleration (early vs. late). T2 and T4 (solid line) share the same acceleration trajectory throughout their duration. Both T2 and T4 reach a peak of acceleration late in the pitch contour; T2′ (dashed line), in contrast, reaches a peak early in the pitch contour.
Iterated rippled noise (IRN) was used to create these stimuli by applying procedures that generate dynamic, curvilinear pitch patterns (Swaminathan et al., 2008). They were generated by applying polynomial equations (Supplementary material, text). A high iteration step (n = 32) was chosen because pitch salience does not increase by any noticeable amount beyond this number of iteration steps. The gain was set to 1. By using IRN, we utilize pitch specific stimuli that preserve dynamic variations in pitch of auditory stimuli that lack a waveform periodicity, formant structure, temporal envelope, and recognizable timbre characteristic of speech. Each stimulus condition consisted of two segments (crossfaded with 5ms cos2 ramps): an initial 500 ms noise segment followed by a 250 ms pitch segment, i.e., T2, T2′, and T4 (Fig. 1, top panel; Supplementary material, audio). The overall root-mean-square level of each segment was equated such that there was no discernible difference in intensity between initial and final segments. All stimuli were presented binaurally at 80 dB SPL through magnetically-shielded tubal insert earphones (ER-3A; Etymotic Research, Elk Grove Village, IL, USA) with a fixed onset polarity (rarefaction) and a repetition rate of 0.94/s. Stimulus presentation order was randomized both within and across participants. All stimuli were generated and played out using an auditory evoked potential system (SmartEP, Intelligent Hearing Systems; Miami, FL, USA).
Cortical pitch response acquisition
Participants reclined comfortably in an electro-acoustically shielded booth to facilitate recording of neurophysiologic responses. They were instructed to relax and refrain from extraneous body movement to minimize myogenic artifacts. They were told to ignore the sounds they heard and were encouraged to sleep throughout the duration of the recording procedure. Almost all participants slept through the recording session and were awakened at the end of the session. The EEG was acquired continuously (5000 Hz sampling rate; 0.3 to 2500 Hz analog band-pass) using ASA-Lab EEG system (ANT Inc., The Netherlands) utilizing a 32-channel amplifier (REFA8-32, TMS International BV) and WaveGuard (ANT Inc., The Netherlands) electrode cap with 32-shielded sintered Ag/AgCl electrodes configured in the standard 10–20-montage system. The high sampling rate of 5 kHz was necessary to recover the brainstem frequency following responses (not reported herein) in addition to the relatively slower cortical pitch components. Because the primary objective of this study was to characterize the cortical pitch components, the EEG acquisition electrode montage was limited to 9 electrode locations: Fpz, AFz, Fz, F3, F4, Cz, T7, T8, M1, M2. The AFz electrode served as the common ground and the common average of all connected unipolar electrode inputs served as default reference for the REFA8-32 amplifier. An additional bipolar channel with one electrode placed lateral to the outer canthi of the left eye and another electrode placed above the left eye was used to monitor artifacts introduced by ocular activity. Inter-electrode impedances were maintained below 10 kΩ. For each stimulus, EEGs were acquired in blocks of 1000 sweeps. The experimental protocol took about 2 hours to complete.
Extraction of the cortical pitch response (CPR)
CPR responses were extracted off-line from the EEG files. To extract the cortical pitch response components, EEG files were first down sampled from 5000 Hz to 2048 Hz. They were then digitally band-pass filtered (3–25 Hz, Butterworth zero phase shift filter with 24 dB/octave rejection rate) to enhance the transient components and minimize the sustained component. Sweeps containing electrical activity exceeding ± 50 μV were rejected automatically. Subsequently, averaging was performed on all 8 unipolar electrode locations using the common reference to allow comparison of CPR components at the right frontal (F4), left frontal (F3), right temporal (T8), and left temporal (T7) electrode sites to evaluate laterality effects. The re-referenced electrode site, Fz-linked T7/T8, was used to characterize the transient pitch response components. This electrode configuration was exploited to improve the signal-to-noise ratio of the CPR components by differentially amplifying (i) the non-inverted components recorded at Fz and (ii) the inverted components recorded at the temporal electrode sites (T7 and T8). This identical electrode configuration makes it possible for us to compare these CPR responses with brainstem responses in subsequent experiments. For both averaging procedures, the analysis epoch was 1200 ms including the 100 ms pre-stimulus baseline.
Analysis of CPR
The evoked response to the entire three segment (noise-pitch-noise) stimulus is characterized by obligatory components (P1/N1) corresponding to the onset of energy in the precursor noise segment of the stimulus followed by several transient CPR components occurring after the onset of the pitch-eliciting segment of the stimulus and an offset component following the offset of the last noise segment in the stimulus. To characterize those attributes of the pitch patterns that are being indexed by the components of the CPR (e.g., pitch onset, pitch acceleration), we evaluated only the latency and magnitude of the CPR components. Peak latencies of response components (Na, Pb, Nb: time interval between pitch-eliciting stimulus onset and response peak of interest) and interpeak latency (Na-Pb, Pb-Nb: time interval between response peaks) were measured to enable us to identify the components associated with pitch onset, pitch acceleration, pitch direction, and stimulus offset. Peak-to-peak amplitude of Na-Pb and Pb-Nb was measured to determine whether variations in amplitude indexed specific aspects of the pitch contour (i.e., pitch acceleration and/or direction). In addition, peak-to-peak amplitude of Na-Pb and Pb-Nb was measured separately at the frontal (F3/F4) and temporal (T7/T8) electrode sites to evaluate laterality effects. To enhance visualization of the laterality effects along a spectrotemporal dimension, a joint time frequency analysis using a continuous wavelet transform was performed on the grand average waveforms derived from the frontal and temporal electrodes. The obligatory onset responses to the noise precursor were not analyzed because they were invariant across the three stimuli.
Statistical analysis
Separate mixed model ANOVAs (SAS®; SAS Institute, Inc., Cary, NC, USA) were conducted on peak latency and peak-to-peak amplitude of the CPR components derived from the Fz electrode site, and peak-to-peak amplitude derived from the T7/T8 and F3/F4 electrode sites. At the Fz electrode site, two-way ANOVAs were performed separately for each component on peak latency and peak-to-peak amplitude to assess language group (Chinese, English) and stimulus (T2, T2′, T4) effects. In the analysis of peak latency, there were three components (Na, Pb, Nb); and in the analysis of peak-to-peak amplitude, two components (Na-Pb, Pb-Nb). At the T7/T8 and F3/F4 electrode sites, three-way (group, stimulus, hemisphere) mixed model ANOVAs were conducted separately on peak-to-peak amplitude of Na-Pb and Pb-Nb. Language group (Chinese, English) was treated as a between-subjects factor and subjects as a random factor nested within group; stimulus (T2, T2′, T4) and hemisphere (T7/T8, F3/F4) were treated as within-subject factors. A priori and post hoc multiple comparisons were corrected with a Bonferroni adjustment at α = 0.05. Where appropriate, partial eta-squared ( ) values were reported to indicate effect sizes.
Results
Response morphology of CPR components
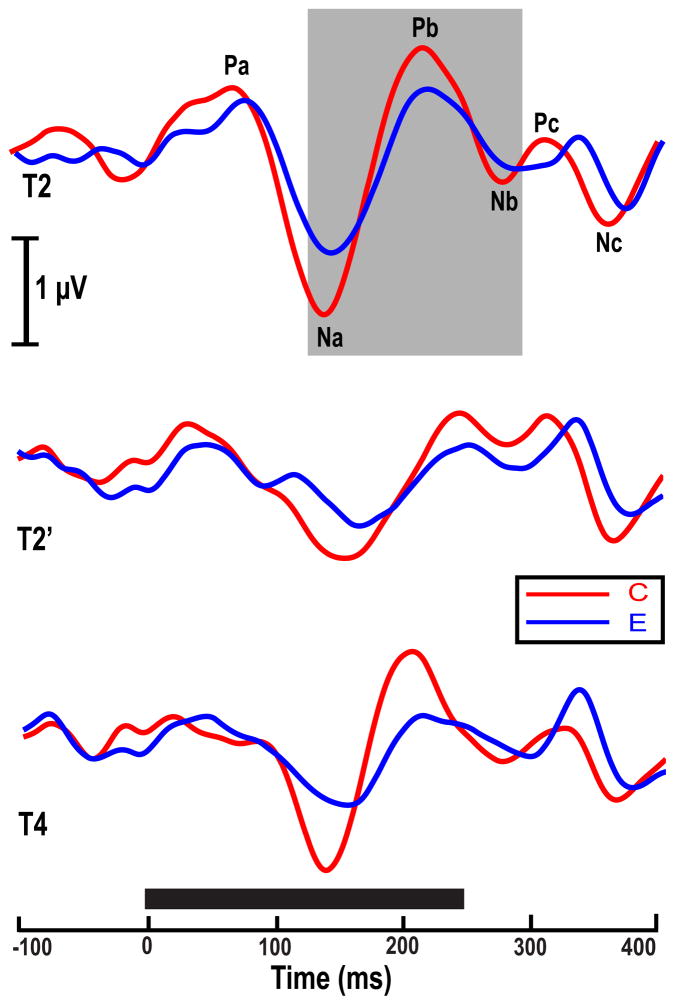
Grand averaged cortical pitch response waveforms to the three stimuli are shown for the Chinese (red trace) and the English (blue trace) group in Fig. 2. CPR components (gray background) are clearly identifiable in both groups. The amplitude of the pitch-relevant components (Na, Pb, Nb) appears to be more robust for the Chinese group especially in response to T2 and T4. The larger amplitude for these two stimuli, which are exemplary representations of lexical tones, but not T2′, may be attributed to changes in location of peak acceleration and language-dependent sensitivity to specific acoustic attributes associated with pitch processing.
Figure 2.
Grand average waveforms of the Chinese and English groups at the Fz electrode site per stimulus condition (T2, T2′, T4). Na, Pb, and Nb (highlighted in gray in the top panel) are the most robust pitch-relevant components. CPR waveforms elicited by the three stimuli show that amplitude of the pitch-relevant components (Na, Pb, Nb) appear to be more robust for the Chinese group especially in response to pitch stimuli with a late acceleration peak (T2, T4). Solid black horizontal bar indicates the duration of each stimulus.
Fz: latency of CPR components
For both language groups, mean Fz peak latencies of CPR components Na, Pb, and Nb increase systematically across stimuli in temporal order of occurrence (Supplementary material, Results, Table S1, Fig. S1a-b; cf. T7/T8 and F3/F4, respectively, in Figs. S2 and S3). As reflected by Na, the omnibus ANOVA yielded a group x stimulus interaction (F2,52 = 5.89, P = 0.0049, ). By group, T2′, the pitch stimulus exhibiting an early peak of acceleration, elicited longer peak latencies than either T2 (C: t52 = −3.21, P = 0.0069; E: t52 = −7.89, P < 0.0001) or T4 (C: t52 = 5.18, P < 0.0001; E: t52 = 6.40, P < 0.0001) in both groups. By stimulus, response peak latencies for T2′ (t52 = −6.29, P < 0.0001) and T4 (t52 = −5.16, P < 0.0001) were longer in the English than the Chinese group. As reflected by Pb, the ANOVA yielded a main effect of stimulus (F2,52 = 100.07, P < 0.0001, ). Both groups exhibited a longer latency evoked by T2′ than either T2 or T4. This means that regardless of language experience, listeners exhibited longer latency to a pitch contour with an early acceleration peak (T2′) relative to those with a late peak (T2, T4). As indexed by Nb, the ANOVA yielded a main effect of group (F1,26 = 10.42, P = 0.0034, ). The longer latency in the English group, relative to the Chinese, was evoked across stimuli.
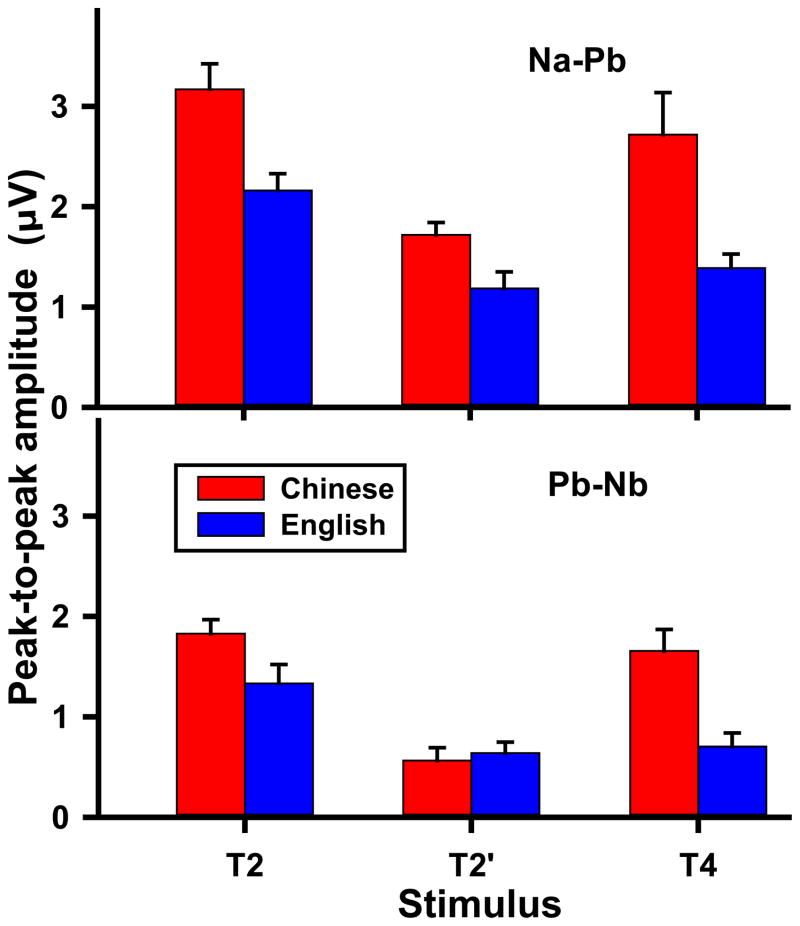
Fz: amplitude of CPR components
Fig. 3 displays group and stimulus effects on peak-to-peak amplitude of Na-Pb and Pb-Nb in response to all three stimuli (T2, T2′, T4). For Na-Pb (top panel), an omnibus ANOVA revealed main effects of group (F1,26 = 18.73, P = 0.0002, ) and stimulus (F2,52 = 15.99, P < 0.0001, ). Pooled across stimuli, Chinese exhibited greater amplitude than English; pooled across groups, T2 and T4 were greater than T2′, T2 was greater than T4. In the absence of a group × stimulus interaction, these differences may be attributed to acoustic properties of the stimuli: auditory sensitivity to location of peak acceleration (T2, T4 > T2′) and pitch direction (T2 > T4). For Pb-Nb (bottom panel), an omnibus ANOVA showed a significant group × stimulus interaction (F2,52 = 6.32, P = 0.0035, ). By group, post hoc comparisons indicated that Chinese exhibited greater amplitude in response to T2 (t52 = 6.17, P < 0.0001) and T4 (t52 = −5.53, P < 0.0001) as compared to T2′; in contrast, English showed greater amplitude to T2 than either T2′ (t52 = 3.38, P = 0.0042) or T4 (t52 = −5.53, P < 0.0001). This disparity in stimulus patterns between Chinese and English indicates that stimulus properties alone are insufficient to explain this language-dependent effect. We suggest that the dominant experience-dependent enhancement of the Chinese Pb-Nb component to native lexical tones T2 and T4 “masks” their differential sensitivity to location of peak acceleration and pitch direction, which is clearly observed for the English for whom there is no experience-dependent enhancement. By stimulus, Chinese amplitude was greater than English in response to T2 (t52 = 2.25, P = 0.0288) and T4 (t52 = 4.31, P < 0.0001), but not T2′. Taken together, these data suggest that early cortical stages of pitch processing are influenced by extrasensory, perceptually-relevant features of speech in one’s native language.
Figure 3.
Mean peak-to-peak amplitude of CPR components Na-Pb (top panel) and Pb-Nb (bottom panel) extracted from Fz electrode site as a function of stimulus. For Na-Pb, Chinese exhibit greater amplitude than English across stimuli; T2 and T4 are greater than T2′ across groups. For Pb-Nb, Chinese listeners show greater amplitude in response to those pitch contours with a late acceleration peak (T2, T4) as compared to T2′ with an early acceleration peak. English listeners, on the other hand, show greater amplitude of T2 relative to T4 in addition to T2′. Error bars = ±1 SE.
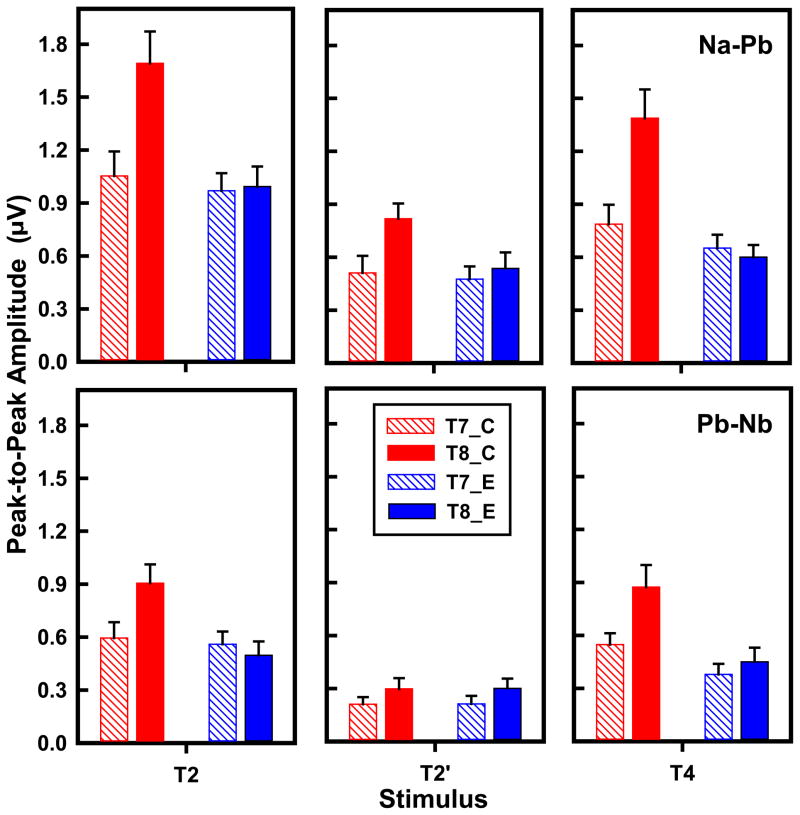
T7/T8 & F3/F4: amplitude of CPR components
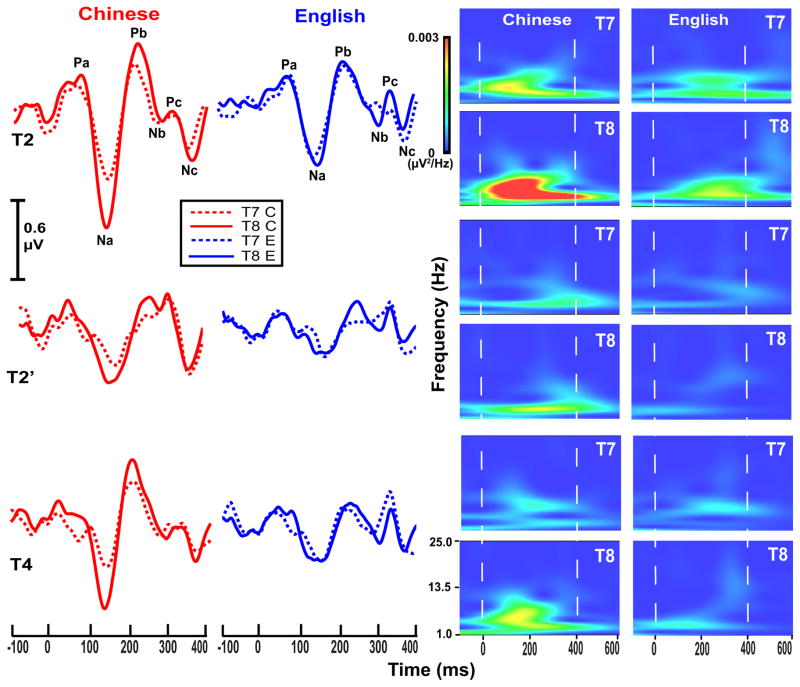
Grand average waveforms of the CPR components for each of the three stimuli per language group (left two columns) and their corresponding spectra (right two columns) are displayed in Fig. 4. CPR components in the Chinese group are greater in magnitude (left) and show a robust right hemisphere preference (right) for T2 and T4 with no discernible hemispheric asymmetry at the F3/F4 electrode sites (Supplementary material, Results, Fig. S4).
Figure 4.
Grand average waveforms (left) and their corresponding spectra (right) of the CPR components for the two language groups (Chinese, English) recorded at electrode sites T7 (dashed) and T8 (solid) for each of the three stimuli (T2, T2′, T4). CPR waveforms appear to show a right-sided preference (T8 > T7) for the Chinese group especially in response to pitch stimuli with a late acceleration peak representative of native lexical tones (T2, T4). The robust rightward preference for T2 and T4 is clearly evident in the spectrotemporal plots. For either group, no hemisphere effects are observed in response to T2′, a pitch pattern that does not occur in any of the world’s tonal inventories. The zero on the x-axis denotes the time of onset of the pitch-eliciting segment of the three stimuli. Na–Pb and Pb–Nb time windows are demarcated by two vertical, white dashed lines.
Group, stimulus, and hemisphere effects on peak-to-peak amplitude of Na-Pb and Pb-Nb are displayed for the temporal sites (T7/T8) in Fig. 5 (Supplementary material, cf. frontal sites F3/F4 in Fig. S2). An omnibus three-way (group ×stimulus × hemisphere) ANOVA on Na-Pb amplitude revealed a significant main effect of stimulus (F2,52 = 27.32, P < 0.0001, ) and a two-way interaction between group and hemisphere (F1,26 = 15.87, P = 0.0005, ). Pooled across group and hemisphere, stimuli varied in magnitude as a function of pitch direction (T2 > T4) and location of peak acceleration (T2, T4 > T2′). Post hoc comparisons at each level of group showed a RH advantage (T8 > T7) for the Chinese group only (t26 = −5.76, P < 0.0001). At each level of hemisphere, a group difference (C > E) in Na-Pb amplitude was restricted to the RH (t26 = 5.01, P < 0.0001). The group × stimulus interaction failed to reach significance.
Figure 5.
Mean peak-to-peak amplitude of CPR components (Na-Pb, top row; Pb-Nb, bottom row) extracted from T7/T8 in the temporal lobe as a function of language group (Chinese, red; English, blue), stimulus (T2, T2′, T4), and hemisphere (left, diagonal; right, solid). Na-Pb amplitude shows a hemispheric asymmetry favoring the RH (T8 > T7) in response to T2 and T4 in the Chinese group only. These same two pitch stimuli also elicit a language group difference (C > E) in the RH only. Both T2 and T4 are larger than T2′ only in the RH for the Chinese group. Pb-Nb amplitude similarly reveals a rightward asymmetry in the Chinese group exclusively as well as Chinese superiority relative to English in the RH. Error bars = ±1 SE. C, Chinese; E, English.
In contrast, results from an omnibus ANOVA on Pb-Nb amplitude yielded significant two-way interaction effects of group × hemisphere (F1,26 = 5.71, P = 0.0245, ) and group × stimulus (F2,52 =3.83, P = 0.0280, ). Post hoc comparisons at each level of group and hemisphere revealed the same pattern as Na-Pb amplitude. Only the Chinese group showed a rightward asymmetry (t26 = −3.90, P = 0.0006); the language group effect was limited to the RH (t26 = 3.88, P = 0.0006). Post hoc comparisons at each level of group and stimulus revealed that Chinese Pb-Nb amplitude was greater than English in response to T2 (t52 = 2.58, P = 0.0127) and T4 (t52 = 3.45, P = 0.0011); and that the amplitude of T2 (t52 = 6.24, P < 0.0001) and T4 (t52 = −5.74, P < 0.0001) was greater than T2′ for the Chinese group only. Important to note is that these stimulus patterns are identical to those obtained from Pb-Nb amplitude at the Fz electrode site. The stimulus patterns of English, on the other hand, differ from those of Chinese irrespective of electrode site: T7/T8 (T2 > T2′, t52 = 3.41, P = 0.0037) or F3/F4 (T2 > T2′, t52 = 3.38, P = 0.0042; T2 > T4, t52 = 3.06, P = 0.0105). In sum, we observe a divergence in stimulus patterns between Chinese and English. We also observe equivalent Nb-Pb responses of Chinese across temporal and frontal electrode sites. These combined findings lead us to suggest that extrasensory and sensory effects are differentially-weighted throughout the time course of the CPR.
Discussion
The major findings of this study demonstrate that pitch-relevant neural activity as reflected in the scalp-recorded CPR components show distinct changes that can be attributed to language-experience (Chinese vs. English); pitch patterns (native vs. nonnative); changes in acoustic attributes (rising vs. falling; and location of peak acceleration (early vs. late) of dynamic, time varying pitch contours. As reflected by the amplitude of the Pb-Nb component at both Fz and temporal (T7/T8) sites, Chinese, unlike English, are greater in response to pitch contours that occur in the Mandarin tonal space as compared to a nonnative pitch contour. As indexed by the amplitude of both components (Na-Pb, Pb-Nb) at temporal sites pooled across stimuli, a rightward asymmetry occurs in the Chinese group only, and moreover, it is only over the right temporal site in which Chinese amplitude is greater than English. These findings suggest that basic neural mechanisms of pitch are sensitive to multiple attributes of dynamic pitch shared in common across languages at early stages of processing in the right auditory cortex. Yet overlaid along the same pitch processing hierarchy are changes that reflect language-dependent modulation of those temporal attributes of pitch contours that provide perceptually-salient cues to tonal recognition in one’s native language.
Differential weighting of sensory and extrasensory effects in early cortical pitch processing
At a neurocomputational level, sensitivity may be manifested by response properties of neural elements including sharper tuning, greater temporal synchronization, and improved synaptic efficiency to enable optimal representation of behaviorally-relevant, dynamic pitch contours. Our experimental paradigm is free of task demands. Stimuli are reduced to the pitch parameter only. Thus, the observed electrophysiological responses are putatively specific to pitch. Our findings converge with an extant literature that attests to the crucial role of the RH in the processing of linguistic as well as nonlinguistic pitch (Zatorre et al., 2002; Friederici & Alter, 2004; Hyde et al., 2008; Meyer, 2008; Zatorre & Gandour, 2008). The preferential recruitment of pitch mechanisms in right auditory cortex by the Chinese group supports the notion that the RH is specifically involved in the analysis of suprasegmental parameters of speech (Friederici, 2011).
The question arises whether the effects of language experience on hemispheric laterality at early cortical stages of processing are driven by extrasensory influences as well as purely acoustic properties of the stimuli. By extrasensory, we mean neural processes at a higher hierarchical level beyond the purely sensory processing of acoustic attributes of the stimulus. One likely candidate for fine-grained stored representations of pitch attributes at this early sensory cortical level of processing is analyzed sensory memory (Cowan, 1984; 1987; cf. Xu et al., 2006). This memory store is to be distinguished from the initial, sensory memory trace and later cognitive processes with their associated memory stores (e.g., short-term memory). It contains analyzed sensory codes including information about pitch height, time-varying pitch direction and acceleration, and timing of pitch onset and offset. In this study, two of the stimuli are exemplary of pitch contours associated with lexical tones (T2, T4); one is not (T2′). As indexed by Na-Pb amplitude over the temporal sites, a rightward asymmetry is limited to the Chinese group. It is over the right temporal site that Chinese Na-Pb amplitude is larger than English. But the group × stimulus interaction is not significant. Therefore, any stimulus effects must be attributed primarily to acoustic properties of the stimuli - location of the acceleration peak (T2, T4: late > T2′: early) and pitch direction (T2: rising > T4: falling). Chinese enhancement of amplitude relative to English can be explained by invoking sensory influences alone or by claiming that sensory influences predominate over extrasensory. However, as indexed by Pb-Nb amplitude over the same temporal sites, the group × stimulus interaction is significant. Chinese Pb-Nb amplitude is greater than English in response to native pitch contours only (T2, T4). Chinese amplitude of T2 and T4 is greater than T2′, not so for English. We argue that this experience-dependent effect demonstrates that extrasensory components may predominate over sensory components in their influence within a given temporal integration window or, in other words, mask purely sensory effects. If purely sensory, then we cannot account for why we do not observe the same stimulus pattern in the English group.
We expect extrasensory influences to target especially those pitch attributes that are perceptually salient in a particular language. It is not accidental that extrasensory effects emerge in Pb-Nb instead of Na-Pb. In a behavioral experiment using excised segments from F0 contours of Mandarin tones (Whalen & Xu, 1992), tonal recognition is shown to be markedly better in the later segments of portions of Tone 2 (rising) and Tone 4 (falling). It is precisely those portions that coincide with a large change in F0. More recently, analysis of brainstem responses in both speech and nonspeech contexts reveals that pitch representations are stronger in Chinese than English in the later, rapidly-changing portions of Tone 2 and Tone 4 (Krishnan et al., 2009a; Krishnan et al., 2009b). Though we are unable to match up portions of F0 contours with CPR components in the current experimental design, we speculate that Pb-Nb is targeting those same perceptually relevant portions of T2 and T4.
As indexed by either Na-Pb or Nb-Pb amplitude over the frontal electrode sites (F3/F4), no hemispheric preferences are evoked by any of the three stimuli irrespective of language group. Though Chinese amplitude is greater than English, stimulus patterns are similar to those over the temporal sites (T2, T4 > T2′; T2 > T4). In the absence of a significant group x stimulus interaction, these stimulus effects can be explained simply on physical properties of the stimuli (location of acceleration peak and pitch direction). This disparity between the temporal and frontal sites is consistent with extant literature that identifies the right auditory cortex as playing a critical role in early stages of pitch processing. Similar to the RH preference for processing linguistic pitch as reflected by the CPR, a RH preference has also been reported for processing the more salient, consonantal musical stimuli in musicians using the pitch onset response (Bidelman & Grall, 2014). These findings taken together suggest that the RH is preferentially recruited for optimal representation of pitch attributes that are perceptually relevant regardless of the domain in which they are presented.
Effects of acoustic properties of stimuli in early cortical pitch processing
Our findings on Fz peak latency of CPR components point to effects on the temporal integration window. Regardless of group, T2′ elicited a longer latency than T2 or T4 as indexed by components Na and Pb. A longer temporal integration window for T2′ likely reflects decreased temporal sensitivity and/or neural desynchronization to a rapidly rising portion of the pitch contour that occurs early as compared to T2 and T4 in which the rapidly gliding portion occurs much later. In the case of Na, English listeners show a longer latency than Chinese in response to T2′ and T4. These findings together suggest that both the location of acceleration peak and pitch direction may differentially affect the duration of the temporal integration window.
Our findings on Fz peak-to-peak amplitude of CPR components reveal that Chinese exhibit greater sensitivity to both pitch direction and location of acceleration peak. As indexed by Na-Pb, the rising pitch contour (T2) evokes larger amplitude than the falling (T4) across language groups. Yet both stimuli share in common a late acceleration peak. This differential sensitivity to pitch direction is supported at multiple levels of the auditory system by various experimental techniques (human psychophysical, multidimensional scaling, electrophysiological, cochlear microphonics, 8th nerve compound action potentials, and responses of the ventral cochlear nucleus; see Supplementary material, Discussion, for details). T2′ is also a rising pitch contour, yet it does not exhibit larger amplitude than T4. Its absence is likely due to biomechanical constraints on the velocity of laryngeal movements in tone production (Erickson, 1976; Ohala, 1978; Xu & Sun, 2002). It cannot be due to differences in acceleration rate per se because T2 and T2′ share an identical acceleration trajectory. It must therefore be attributed to its early location near the onset of T2′ that likely reflects differences in neural synchronization when a rapid rise in pitch occurs earlier as compared to later in the pitch contour. Differences in pitch direction notwithstanding, the two stimuli with a late acceleration peak (T2, T4) have larger amplitude than T2′.
As indexed by Pb-Nb, we found an interaction (group × stimulus) that reveals differential weighting of sensory and extrasensory components depending upon one’s language experience. In the Chinese group, the two lexical tones (T2, T4) have greater amplitude than T2′. In contrast, T2 amplitude is greater than T2′ and T4 in the English group. The English pattern may be attributed to differences in auditory sensitivity to pitch direction (T2 > T4) and location of peak acceleration (T2 > T2′). Although the Chinese pattern similarly shows auditory sensitivity to location of peak acceleration, this specific pitch attribute also segregates native lexical tones (T2, T4) from a nonnative pitch stimulus (T2′). If strictly sensory, one would expect parallel stimulus patterns irrespective of language experience. To the contrary, we observe that Chinese amplitude is greater than English in response to just those pitch contours representative of Mandarin tones. The lack of a language group effect in response to T2′ is in agreement with previous studies which similarly failed to show experience-dependent enhancement of pitch-relevant neural activity for nonnative pitch contours at the level of the cerebral cortex (Chandrasekaran et al., 2009, T2′; Krishnan et al., 2014, flat & linear rising ramp) and at the level of the auditory brainstem (Krishnan et al., 2009a, linear rising & trilinear rising ramps, T2′).
These findings can be accounted for by invoking the influence of extrasensory effects on pitch processing that are associated with perceptually-relevant features of Mandarin lexical tones. Even though T2 (late acceleration peak) elicits larger amplitude than T2′ (early acceleration peak) in both groups, the amplitude of T2 is still larger in the Chinese group. This finding suggests that the fundamental neural mechanism is the same for Chinese and English listeners alike, but Chinese are more sensitive to pitch attributes that are behaviorally-relevant for pitch processing because of their long-term experience with a tonal language. Because enhanced sensitivity to time-varying dimensions of pitch (e.g., acceleration) is already present in neural activity at the level of the brainstem (Krishnan & Gandour, 2009; Krishnan et al., 2012, reviews), it seems plausible that cortical pitch mechanisms may be reflecting, at least in part, this enhanced pitch input from the brainstem (Bidelman et al., 2014).
Predictive coding may underlie experience-dependent processing of pitch in the auditory cortex
Growing evidence suggests that pitch-relevant information is available in primary and non-primary areas of auditory cortex: functional imaging plus direct cortical recording (Patterson et al., 2002; Penagos et al., 2004; Griffiths et al., 2010; Puschmann et al., 2010), patients with focal excisions (Zatorre, 1988; Zatorre & Samson, 1991; Johnsrude et al., 2000), and magnetoencephalography (Gutschalk et al., 2002; Krumbholz et al., 2003; Gutschalk et al., 2004). Lateral HG also appears to be important for computations relevant to extraction of pitch of complex sounds (Zatorre & Belin, 2001; Hall et al., 2002; Schonwiesner et al., 2005).
A hierarchical processing framework for coordinated interaction between these areas is provided by application of predictive coding model of perception to depth-electrode recordings of pitch-relevant neural activity along HG (Rao & Ballard, 1999; Kumar et al., 2011; Kumar & Schonwiesner, 2012). Essentially, higher-level areas in the hierarchy contributing to pitch (lateral HG) use stored information of pitch to make a pitch prediction. This prediction is passed to the lower areas in the processing hierarchy (medial and middle HG) via top down connection(s). The lower areas then compute a prediction error. The strength of the top-down and bottom-up connections is continually adjusted in a recursive manner in order to minimize predictive error and to optimize representation at the higher level. Consistent with the predictions of the model, Kumar et al. (2011) showed that strength of connectivity varies with pitch salience such that the strength of the top down connection from lateral HG to medial and middle HG increased with pitch salience, whereas the strength of the bottom up connection from middle HG to lateral HG decreased. It is likely that lateral HG has more pitch-specific mechanisms, and therefore plays a relatively greater role in pitch perception.
Applied to our data, this framework suggests that CPR changes attributable wholly to acoustic properties of the stimulus invoke a recursive process in the representation of pitch (initial pitch prediction, error generation, error correction). At this level, the hierarchical flow of processing and its connectivity strengths along the HG are essentially the same regardless of one’s language background. However, the initial pitch prediction at the level of the lateral HG is more precise for Chinese because of their access to stored information about T2 and T4 with a smaller error term. Consequently, the top-down connection from lateral HG to medial and middle HG is stronger than the bottom-up connection. The opposite would be true for English because of their less precise initial prediction. Language experience therefore alters the nature of the interaction between levels along the hierarchy of pitch processing by modulating connection strengths.
Pitch processing in the auditory cortex is also influenced by inputs from subcortical structures that are themselves subject to experience-dependent plasticity. It is likely that top-down connections in the hierarchy provide feedback to adjust the effective time scales of processing at each stage to optimally control the temporal dynamics of pitch processing (Balaguer-Ballester et al., 2009). This expanded model represents a unified, physiologically plausible, theoretical framework that includes both cortical and subcortical components in the hierarchical processing of pitch.
Conclusions
CPR components provide a series of robust neurobiological markers that reveal differential sensitivity to language-universal (acoustic) and overlaid language-dependent (linguistic) temporal attributes of pitch processing during early sensory level processing in the auditory cortex. Enhancement of native pitch stimuli and stronger rightward asymmetry of CPR components in the Chinese group is consistent with the notion that long-term experience shapes adaptive, distributed hierarchical pitch processing in the auditory cortex, and reflects an interaction with higher-order, extrasensory processes beyond the sensory memory trace. Within a given temporal integration window, pitch processing involves a hierarchy of both sensory and extrasensory effects whose relative weighting varies depending on language experience.
Supplementary Material
Acknowledgments
Research supported by NIH 5R01DC008549 (A.K.). Thanks to Rongrong Wang and Longjie Cheng for their assistance with statistical analysis (Department of Statistics); Breanne Lawler, Kate Geisen and Jilian Wendel for their help with data acquisition; Venkatakrishnan Vijayaraghavan with computer programming.
Abbreviations
- ANOVA
analysis of variance
- C
Chinese
- E
English
- CPR
cortical pitch response
- EEG
electroencephalography
- HG
Heschl’s gyrus
- IRN
iterated rippled noise
- MMN
mismatch negativity
- RH
right hemisphere
- T2
Mandarin Tone 2
- T2′
flipped variant of Mandarin Tone 2
- T4
Mandarin Tone 4
Footnotes
The authors declare no conflict of interest.
Additional supporting information can be found in the online version of this article:
Stimuli: Equations to generate the IRN stimuli (T2, T2′, T4)
Media files: Audio of IRN stimuli (irnpitchT2.mp3; irnpitchT2′.mp3; irnpitchT4.mp3) and stimulus condition (irnnoisetopitchT2.mp3)
Table S1: Mean peak latencies of CPR components Na, Pb, and Nb derived from Fz electrode site
Figures S1a & S1b: Mean peak latencies of CPR components Na, Pb, and Nb derived from Fz electrode site
Figure S2: Mean peak latencies of CPR components Na, Pb, and Nb derived from T7/T8 electrode sites
Figure S3: Mean peak latencies of CPR components Na, Pb, and Nb derived from F3/F4 electrode sites
Figure S4: Grand average waveforms and their corresponding spectra of CPR components extracted from F3/F4
Figure S5: Mean peak-to-peak amplitude of CPR components extracted from F3/F4
Discussion: Effects of acoustic properties of stimuli in early cortical pitch processing
Contributor Information
Ananthanarayan Krishnan, Email: rkrish@purdue.edu.
Jackson T. Gandour, Email: gandour@purdue.edu.
Chandan H. Suresh, Email: hs0@purdue.edu.
References
- Alho K, Grimm S, Mateo-Leon S, Costa-Faidella J, Escera C. Early processing of pitch in the human auditory system. Eur J Neurosci. 2012;36:2972–2978. doi: 10.1111/j.1460-9568.2012.08219.x. [DOI] [PubMed] [Google Scholar]
- Balaguer-Ballester E, Clark NR, Coath M, Krumbholz K, Denham SL. Understanding pitch perception as a hierarchical process with top-down modulation. PLoS Comput Biol. 2009;5:e1000301. doi: 10.1371/journal.pcbi.1000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidelman GM, Grall J. Functional organization for musical consonance and tonal pitch hierarchy in human auditory cortex. Neuroimage. 2014;101:204–214. doi: 10.1016/j.neuroimage.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Bidelman GM, Weiss MW, Moreno S, Alain C. Coordinated plasticity in brainstem and auditory cortex contributes to enhanced categorical speech perception in musicians. Eur J Neurosci. 2014;40:2662–2673. doi: 10.1111/ejn.12627. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran B, Krishnan A, Gandour JT. Sensory processing of linguistic pitch as reflected by the mismatch negativity. Ear Hear. 2009;30:552–558. doi: 10.1097/AUD.0b013e3181a7e1c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N. On short and long auditory stores. Psychol Bull. 1984;96:341–370. [PubMed] [Google Scholar]
- Cowan N. Auditory sensory storage in relation to the growth of sensation and acoustic information extraction. J Exp Psychol Hum Percept Perform. 1987;13:204–215. doi: 10.1037//0096-1523.13.2.204. [DOI] [PubMed] [Google Scholar]
- Erickson D. A physiological analysis of the tones of Thai. University of Connecticut; 1976. [Google Scholar]
- Friederici AD. The brain basis of language processing: from structure to function. Physiol Rev. 2011;91:1357–1392. doi: 10.1152/physrev.00006.2011. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Alter K. Lateralization of auditory language functions: a dynamic dual pathway model. Brain Lang. 2004;89:267–276. doi: 10.1016/S0093-934X(03)00351-1. [DOI] [PubMed] [Google Scholar]
- Griffiths TD, Kumar S, Sedley W, Nourski KV, Kawasaki H, Oya H, Patterson RD, Brugge JF, Howard MA. Direct recordings of pitch responses from human auditory cortex. Curr Biol. 2010;20:1128–1132. doi: 10.1016/j.cub.2010.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutschalk A, Patterson RD, Rupp A, Uppenkamp S, Scherg M. Sustained magnetic fields reveal separate sites for sound level and temporal regularity in human auditory cortex. Neuroimage. 2002;15:207–216. doi: 10.1006/nimg.2001.0949. [DOI] [PubMed] [Google Scholar]
- Gutschalk A, Patterson RD, Scherg M, Uppenkamp S, Rupp A. Temporal dynamics of pitch in human auditory cortex. Neuroimage. 2004;22:755–766. doi: 10.1016/j.neuroimage.2004.01.025. [DOI] [PubMed] [Google Scholar]
- Hall DA, Johnsrude IS, Haggard MP, Palmer AR, Akeroyd MA, Summerfield AQ. Spectral and temporal processing in human auditory cortex. Cereb Cortex. 2002;12:140–149. doi: 10.1093/cercor/12.2.140. [DOI] [PubMed] [Google Scholar]
- Howie JM. Acoustical studies of Mandarin vowels and tones. Cambridge University Press; New York: 1976. [Google Scholar]
- Hyde KL, Peretz I, Zatorre RJ. Evidence for the role of the right auditory cortex in fine pitch resolution. Neuropsychologia. 2008;46:632–639. doi: 10.1016/j.neuropsychologia.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Itoh K, Okumiya-Kanke Y, Nakayama Y, Kwee IL, Nakada T. Effects of musical training on the early auditory cortical representation of pitch transitions as indexed by change-N1. Eur J Neurosci. 2012;36:3580–3592. doi: 10.1111/j.1460-9568.2012.08278.x. [DOI] [PubMed] [Google Scholar]
- Johnsrude IS, Penhune VB, Zatorre RJ. Functional specificity in the right human auditory cortex for perceiving pitch direction. Brain. 2000;123:155–163. doi: 10.1093/brain/123.1.155. [DOI] [PubMed] [Google Scholar]
- Koelsch S. Brain & Music. Wiley-Blackwell; Chichester, UK: 2012. [Google Scholar]
- Krishnan A, Gandour JT. The role of the auditory brainstem in processing linguistically-relevant pitch patterns. Brain Lang. 2009;110:135–148. doi: 10.1016/j.bandl.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A, Gandour JT, Ananthakrishnan S, Vijayaraghavan V. Language experience enhances early cortical pitch-dependent responses. J Neurolinguistics. 2015;33:128–148. doi: 10.1016/j.jneuroling.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A, Gandour JT, Bidelman GM. Experience-dependent plasticity in pitch encoding: from brainstem to auditory cortex. Neuroreport. 2012;23:498–502. doi: 10.1097/WNR.0b013e328353764d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A, Gandour JT, Bidelman GM, Swaminathan J. Experience-dependent neural representation of dynamic pitch in the brainstem. Neuroreport. 2009a;20:408–413. doi: 10.1097/WNR.0b013e3283263000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A, Gandour JT, Suresh CH. Cortical pitch response components show differential sensitivity to native and nonnative pitch contours. Brain Lang. 2014;138:51–60. doi: 10.1016/j.bandl.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A, Swaminathan J, Gandour JT. Experience-dependent enhancement of linguistic pitch representation in the brainstem is not specific to a speech context. J Cogn Neurosci. 2009b;21:1092–1105. doi: 10.1162/jocn.2009.21077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumbholz K, Patterson RD, Seither-Preisler A, Lammertmann C, Lutkenhoner B. Neuromagnetic evidence for a pitch processing center in Heschl’s gyrus. Cereb Cortex. 2003;13:765–772. doi: 10.1093/cercor/13.7.765. [DOI] [PubMed] [Google Scholar]
- Kumar S, Schonwiesner M. Mapping human pitch representation in a distributed system using depth-electrode recordings and modeling. J Neurosci. 2012;32:13348–13351. doi: 10.1523/JNEUROSCI.3812-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Sedley W, Nourski KV, Kawasaki H, Oya H, Patterson RD, Howard MA, 3rd, Friston KJ, Griffiths TD. Predictive coding and pitch processing in the auditory cortex. J Cogn Neurosci. 2011;23:3084–3094. doi: 10.1162/jocn_a_00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Ni JT, Li ZH, Li XO, Zhang DR, Zeng FG, Chen L. Opposite patterns of hemisphere dominance for early auditory processing of lexical tones and consonants. Proc Natl Acad Sci U S A. 2006;103:19558–19563. doi: 10.1073/pnas.0607065104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutkenhoner B, Seither-Preisler A, Seither S. Piano tones evoke stronger magnetic fields than pure tones or noise, both in musicians and non-musicians. Neuroimage. 2006;30:927–937. doi: 10.1016/j.neuroimage.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Maess B, Jacobsen T, Schroger E, Friederici AD. Localizing pre-attentive auditory memory-based comparison: magnetic mismatch negativity to pitch change. Neuroimage. 2007;37:561–571. doi: 10.1016/j.neuroimage.2007.05.040. [DOI] [PubMed] [Google Scholar]
- Meyer M. Functions of the left and right posterior temporal lobes during segmental and suprasegmental speech perception. Zeitshcrift fur Neuropsycholgie. 2008;19:101–115. [Google Scholar]
- Moore CB, Jongman A. Speaker normalization in the perception of Mandarin Chinese tones. J Acoust Soc Am. 1997;102:1864–1877. doi: 10.1121/1.420092. [DOI] [PubMed] [Google Scholar]
- Ohala J. The production of tone. In: Fromkin V, editor. Tone: A linguistic survey. Academic Press; New York: 1978. pp. 15–39. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Patel AD. Music, language, and the brain. Oxford University Press; NY: 2008. [Google Scholar]
- Patterson RD, Uppenkamp S, Johnsrude IS, Griffiths TD. The processing of temporal pitch and melody information in auditory cortex. Neuron. 2002;36:767–776. doi: 10.1016/s0896-6273(02)01060-7. [DOI] [PubMed] [Google Scholar]
- Penagos H, Melcher JR, Oxenham AJ. A neural representation of pitch salience in nonprimary human auditory cortex revealed with functional magnetic resonance imaging. J Neurosci. 2004;24:6810–6815. doi: 10.1523/JNEUROSCI.0383-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvermuller F, Shtyrov Y, Hauk O. Understanding in an instant: neurophysiological evidence for mechanistic language circuits in the brain. Brain Lang. 2009;110:81–94. doi: 10.1016/j.bandl.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puschmann S, Uppenkamp S, Kollmeier B, Thiel CM. Dichotic pitch activates pitch processing centre in Heschl’s gyrus. Neuroimage. 2010;49:1641–1649. doi: 10.1016/j.neuroimage.2009.09.045. [DOI] [PubMed] [Google Scholar]
- Rao RP, Ballard DH. Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects. Nat Neurosci. 1999;2:79–87. doi: 10.1038/4580. [DOI] [PubMed] [Google Scholar]
- Schonwiesner M, Rubsamen R, von Cramon DY. Hemispheric asymmetry for spectral and temporal processing in the human antero-lateral auditory belt cortex. Eur J Neurosci. 2005;22:1521–1528. doi: 10.1111/j.1460-9568.2005.04315.x. [DOI] [PubMed] [Google Scholar]
- Swaminathan J, Krishnan A, Gandour JT, Xu Y. Applications of static and dynamic iterated rippled noise to evaluate pitch encoding in the human auditory brainstem. IEEE Trans Biomed Eng. 2008;55:281–287. doi: 10.1109/TBME.2007.896592. [DOI] [PubMed] [Google Scholar]
- Whalen DH, Xu Y. Information for Mandarin tones in the amplitude contour and in brief segments. Phonetica. 1992;49:25–47. doi: 10.1159/000261901. [DOI] [PubMed] [Google Scholar]
- Wong PC, Perrachione TK. Learning pitch patterns in lexical identification by native English-speaking adults. Appl Psycholinguist. 2007;28:565–585. [Google Scholar]
- Xu Y. Contextual tonal variations in Mandarin. J Phonetics. 1997;25:61–83. [Google Scholar]
- Xu Y, Gandour JT, Francis AL. Effects of language experience and stimulus complexity on the categorical perception of pitch direction. J Acoust Soc Am. 2006;120:1063–1074. doi: 10.1121/1.2213572. [DOI] [PubMed] [Google Scholar]
- Xu Y, Sun X. Maximum speed of pitch change and how it may relate to speech. J Acoust Soc Am. 2002;111:1399–1413. doi: 10.1121/1.1445789. [DOI] [PubMed] [Google Scholar]
- Yrttiaho S, Tiitinen H, May PJ, Leino S, Alku P. Cortical sensitivity to periodicity of speech sounds. J Acoust Soc Am. 2008;123:2191–2199. doi: 10.1121/1.2888489. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ. Pitch perception of complex tones and human temporal-lobe function. J Acoust Soc Am. 1988;84:566–572. doi: 10.1121/1.396834. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Belin P. Spectral and temporal processing in human auditory cortex. Cereb Cortex. 2001;11:946–953. doi: 10.1093/cercor/11.10.946. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Belin P, Penhune VB. Structure and function of auditory cortex: music and speech. Trends in cognitive sciences. 2002;6:37–46. doi: 10.1016/s1364-6613(00)01816-7. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Gandour JT. Neural specializations for speech and pitch: moving beyond the dichotomies. Philos Trans R Soc Lond B Biol Sci. 2008;363:1087–1104. doi: 10.1098/rstb.2007.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Samson S. Role of the right temporal neocortex in retention of pitch in auditory short-term memory. Brain. 1991;114 (Pt 6):2403–2417. doi: 10.1093/brain/114.6.2403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.