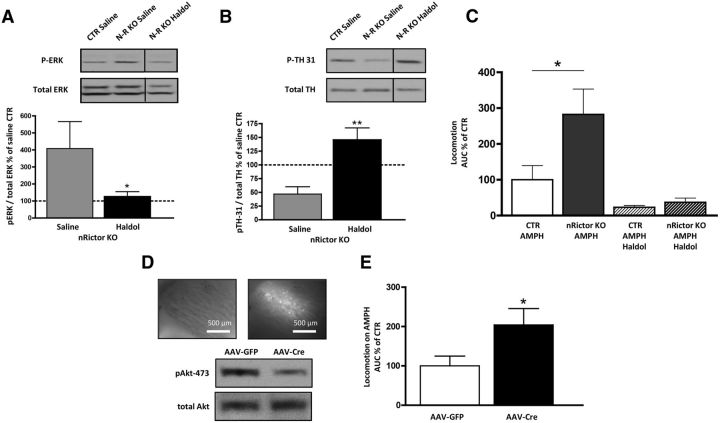
Figure 5.
Treatment with haloperidol supports the role of D2R in exaggerated AMPH-induced hyperactivity in nRictor KO mice, whereas viral intervention reveals that Akt signaling specifically in the dorsal striatum supports this phenotype. A, Acute administration of haloperidol (0.8 mg/kg i.p.) reduced expression of pERK1/2 in the dorsal striatum of nRictor mice. Top, Representative immunoblots for pERK1/2 obtained from striatal tissue of CTR and nRictor mice injected either vehicle or haloperidol (Haldol). Bottom, Data were normalized to saline-injected CTR (n = 9). B, Acute administration of haloperidol (0.8 mg/kg i.p.) decreased expression of pTH-Ser31 in the dorsal striatum of nRictor mice. Top, Representative immunoblots for pTH-Ser31 and total TH obtained from striatal tissue of CTR and nRictor mice injected either vehicle or haloperidol (Haldol). Bottom, Data were normalized to the corresponding total TH and expressed as a percentage of saline-injected CTR (n = 9). C, CTR and nRictor KO mice were habituated to saline injections and open field chambers for 6 d. On day 7, drugs were administered intraperitoneally (AMPH 2 mg/kg; haloperidol 0.8 mg/kg) and horizontal locomotor activity recorded in 5 min intervals. Data are represented as area under the curve from time of injection to 60 min (n = 5–6). D, Viral downregulation of striatal Rictor results in decreased pAkt-473: AAV-Cre-GFP and AAV-eGFP viral vectors were injected into the dorsal striatum of floxed animals. Shown are representative immunoblots of AAV-Cre-injected animals versus AAV-eGFP CTRs. Inset, Representative images (4×) demonstrating GFP in the targeted brain region: white light on the left; FITC filter on the right. E, AMPH (2 mg/kg i.p.) induced locomotion measured following the same handling/habituation protocol as was used for the genetic KO. Data are represented as area under the curve from the time of injection to 60 min (n = 7). *p < 0.05 (t test). **p < 0.01 (t test).