Abstract
Objectives
The spontaneous spinal epidural hematoma (SSEH) is a rare clinical entity. Patients typically present with sudden onset back pain followed by neurological deficits.
Methods
Diagnosis of SSEH is usually made with MRI and standard treatment is surgical evacuation. In 1996, Groen published the most comprehensive review on the SSEH in which he analyzed 333 cases. We review 104 cases of SSEH presented in the English literature since the last major review and add three of our own cases, for a total of 107 cases.
Results
Our patients presented with back pain and neurologic deficits. Two made excellent functional recovery with prompt surgical decompression while one continued to have significant deficits despite evacuation. Better postoperative outcome was associated with less initial neurological dysfunction, shorter time to operation from symptom onset and male patients.
Conclusion
We discuss the etiology of SSEH and report current trends in diagnosis, treatment, and outcome.
Keywords: Spinal epidural hematoma, Back pain, Neurological deficits, MRI
INTRODUCTION
1. Case 1
1) History
The patient is a 71 year-old right handed man who presented to the emergency room with sudden onset of upper back pain, just below his neck, associated with right arm weakness. Within hours, he developed weakness of his legs associated with paresthesia that progressed to paralysis of bilateral lower extremities. Past medical history is significant for arthritis, restless leg syndrome diagnosed six days prior to presentation, and history of syncope.
2) Examination
He had 4+/5 strength in the left upper extremity and 4/5 strength in the right upper extremity with complete paralysis of bilateral lower extremities. Although sensation to light touch was preserved, he had decreased pin-prick sensation and proprioception starting at the cervicothoracic level, worse on the right side than the left. Deep tendon reflexes were brisk and the Babinski reflexes were up-going. He had normal coagulation values.
3) Imaging
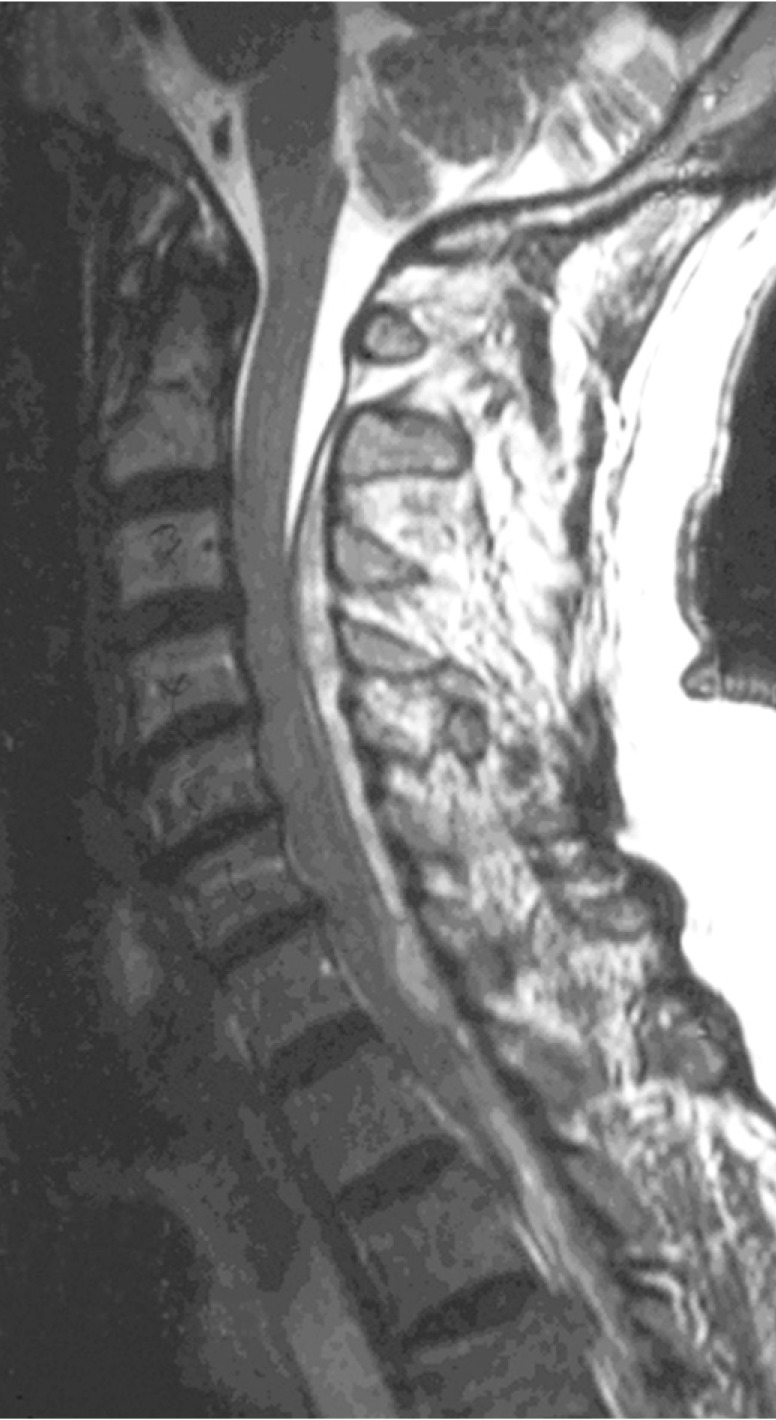
MRI showed large posterolateral extradural mass extending from C2 to T4 with marked spinal cord compression. T1-weighted images showed low signal intensities and T2-weighted images showed heterogeneous intermediate to high signal intensities. No contrast enhancement was found except for a small nodular enhancing focus at the C7-T1 junction to the right of the midline (Fig. 1).
Fig. 1. T2-weighted sagittal magnetic resonance (MR) image showing large posterolateral extradural mass extending from C2 to T4 with marked spinal cord compression.
4) Operation
The patient was taken urgently to the operating room for a C3 to T1 laminectomy. A dark blood clot was noted in the epidural space and was evacuated. Portions of the epidural clot were sent to pathology whose report confirmed the presence of hematoma with no evidence of vascular malformation.
5) Postoperative Course
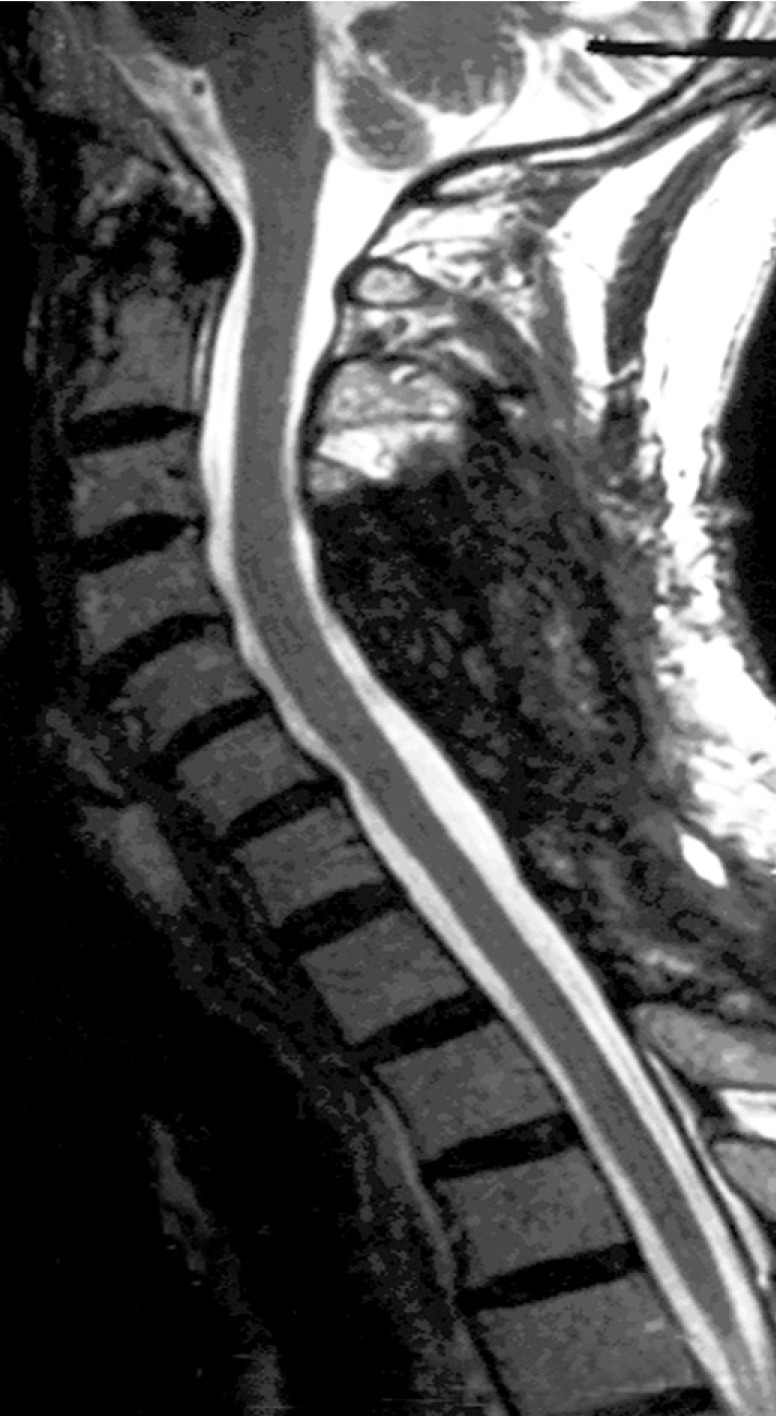
Postoperative MRI showed complete resolution of the hematoma (Fig. 2). The patient recovered well with prompt return of neurologic function. On two-year follow up, he was doing well with no residual neurological deficits, only occasional spasms in his trapezius muscle.
Fig. 2. Postoperative magnetic resonance image shows complete resolution of the hematoma.
1. Case 2
1) History
Patient is a 78 year-old woman who awoke early in the morning with low back pain. Soon afterwards, she presented to the emergency room with additional complaint of right lower extremity pain and weakness with difficulty ambulating. Her past medical history was significant for hypertension, type II diabetes and distant history of TB, previously treated. She had normal coagulation values.
2) Examination
On neurological examination, she had 4/5 right dorsiflexion weakness but otherwise had good motor strength. She had diminished sensation to light touch in her right lower extremity.
3) Imaging
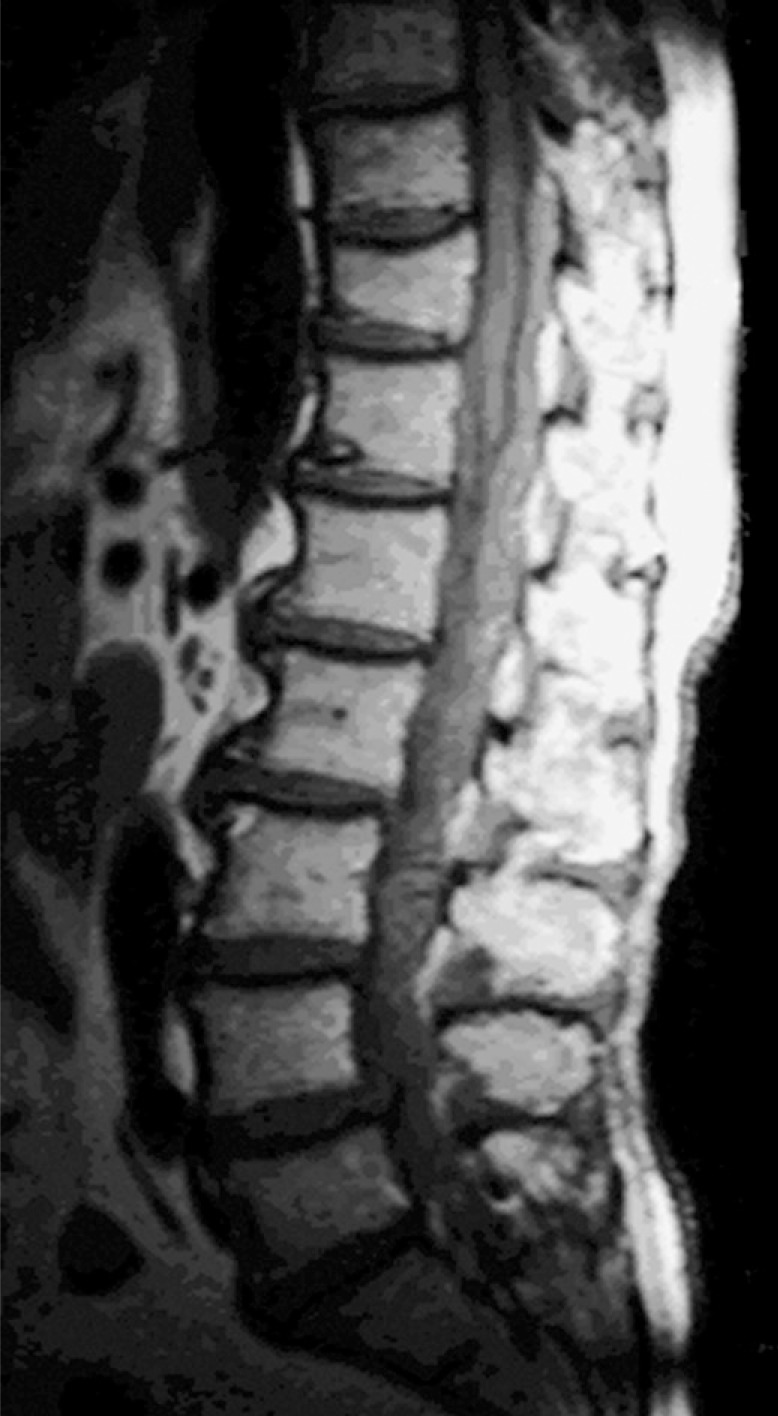
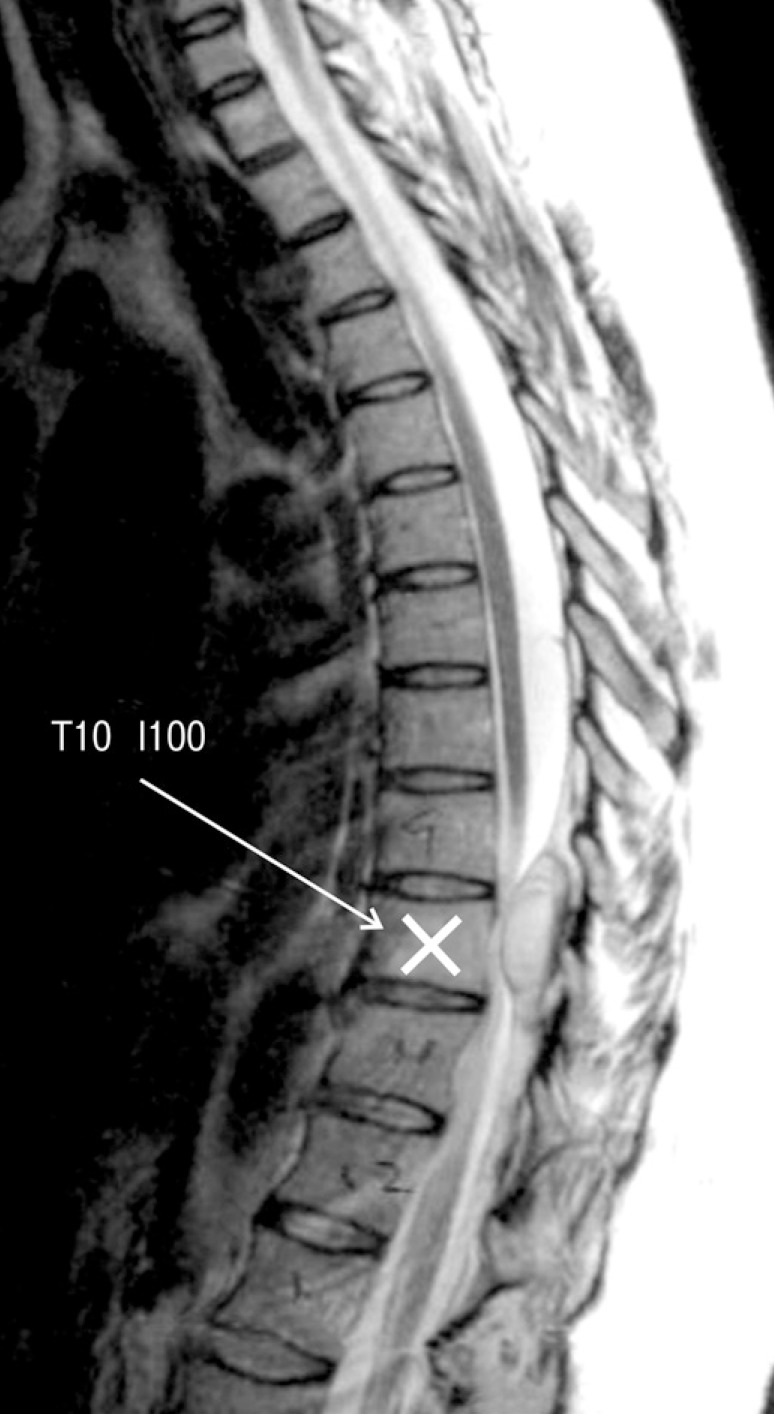
Initial MRI showed epidural mass from T10 to L4 (Fig. 3).
Fig. 3. T1-weighted sagittal MR image showing epidural mass from T10 to L4.
4) Operation
Patient was taken to the operating room 16 hours after the initial symptom of low back pain. She underwent T10 to L4 laminectomy and was noted to have significant compression of the thecal sac from epidural blood clot. The hematoma was evacuated and portions of the specimen were sent to pathology, where findings were consistent with hematoma with no evidence of vascular malformation.
5) Postoperative Course
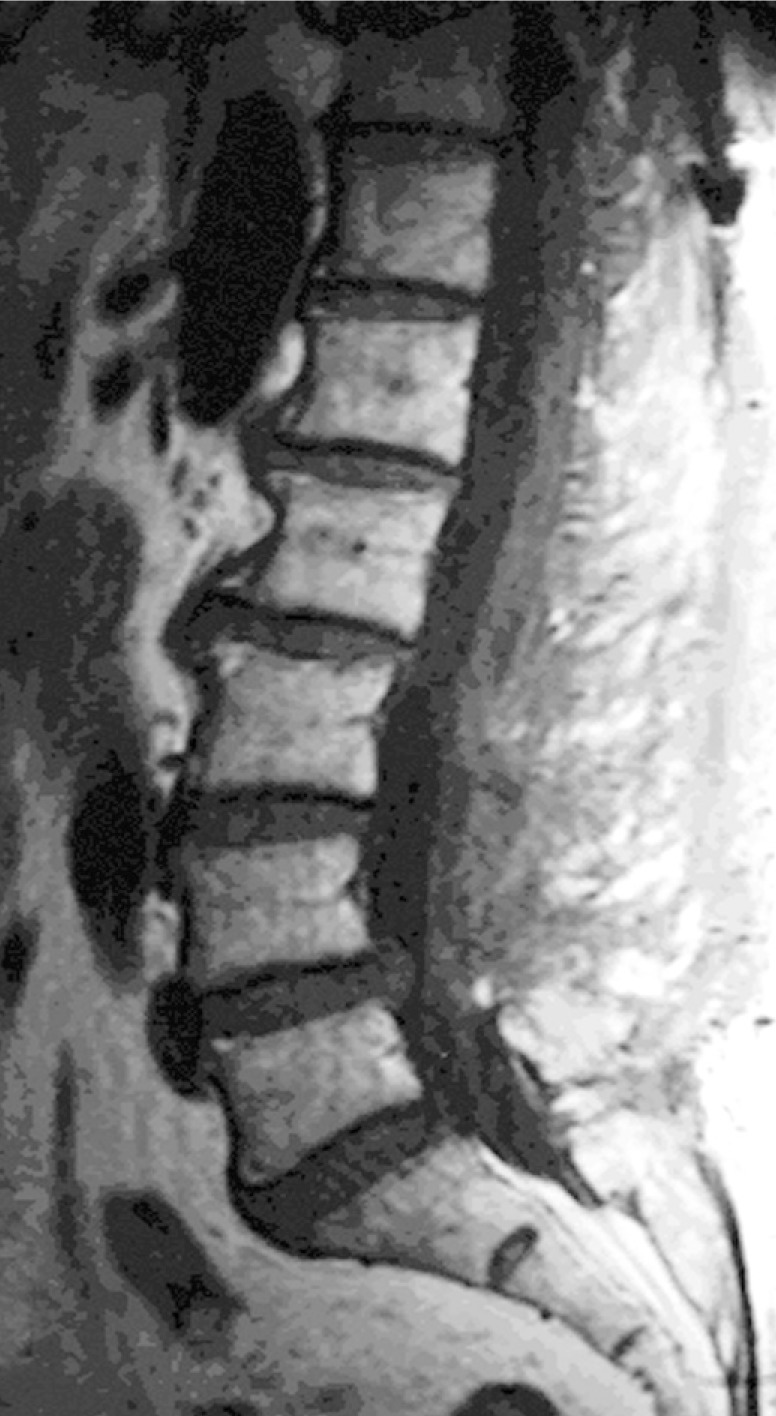
Postoperative MRI showed complete resolution of the hematoma (Fig. 4). Patient showed progressive improvement and eventually gained return of full strength in her right leg. She however, continued to have right leg pain and numbness
Fig. 4. Postoperative MR image revealed complete resolution of the hematoma.
3. Case 3
1) History
A 42 year old woman was gardening when she suddenly developed severe low back pain with nausea. The day prior she had been engaged in heavy physical work. Soon after lying down she noticed she could not move her legs and could not feel anything from the waist down. Past medical history is significant for Aspirin use.
2) Examination
Both legs were completely flaccid. There were absent reflexes in her legs. She also could not feel anything below the T11 sensory level. She had urinary retention as well.
3) Imaging
MRI showed an extradural mass compressing the spinal cord from T9 to T11. It was hyperintense to the spinal cord on T2 imaging. The signal was heterogeneous in character (Fig. 5).
Fig. 5. T2-weighted sagittal MR image shows an extradural mass compressing the spinal cord from T9 to T11.
4) Operation
She had a T9 to L1 laminectomy performed within 24 hours of symptom onset. The hematoma was found to extend down to L1 intraoperatively. Pathological analysis revealed only blood clot.
5) Postoperative Course
The patient had no improvement. She remained paraplegic and has made little improvement with rehabilitation.
MATERIALS AND METHODS
The last major review on SSEH was reported by Groen in 199641). Therefore, our literature search was limited to cases reported since 1996 coinciding with the widespread acceptance and use of MRI. A PubMed literature search for "spontaneous spinal epidural hematoma" was conducted and the reports from the English literature were collected. Hematomas associated with vascular malformations, spinal surgery, trauma, spinal tumors, or any spinal procedures such as diagnostic lumbar punctures were excluded. Patients on anti-coagulation medication and those with hypertension were included since they were part of the past reviews on SSEH. The diagnosis was made with an imaging study and confirmed in most cases by surgery.
We statistically investigated the effect of time to surgery (before or after 24 hours of hospital admission) on neurological outcome. Because the literature accounts were inconsistent in their clinical descriptions of initial and final neurological status, the Frankel grading system was used to provide a standardized assessment of neurological dysfunction. The initial and final Frankel grade for each patient was assigned based on symptomatic reports (Table 1). For quantitative analysis, the Frankel grade scale of A-E was converted to a 1-5 scale, respectively. We used linear regression to investigate the relationship between final Frankel grade and time to surgery. Initial Frankel grade, age, sex, time to surgery, size of hematoma, history of hypertension, and use of anticoagulants were included as covariates. We first considered the effects of time to surgery, initial Frankel grade, age, and sex and then sequentially added two-way interactions as well as effects associated with size of hematoma, hypertension and use of anticoagulants. Four age categories were used: less than 20 years, 20 to 40 years, 40 to 60 years and 60 years of age and older. Statistical analyses were conducted using R version 2.12.1 and a significance level of 0.05 was used to determine significant effects.
Table 1. Frankel grade system.
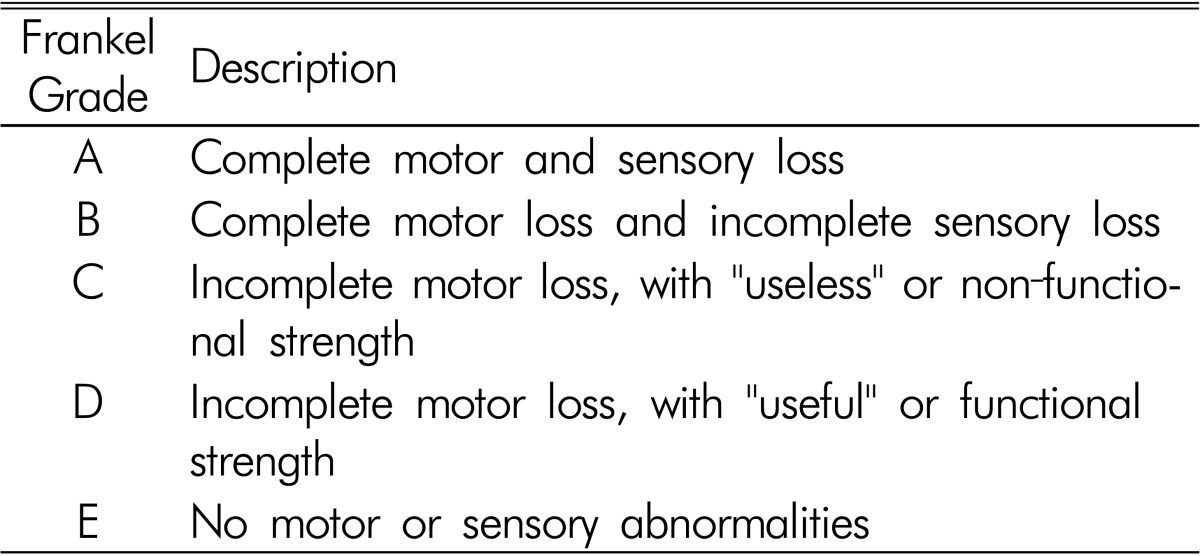
RESULTS
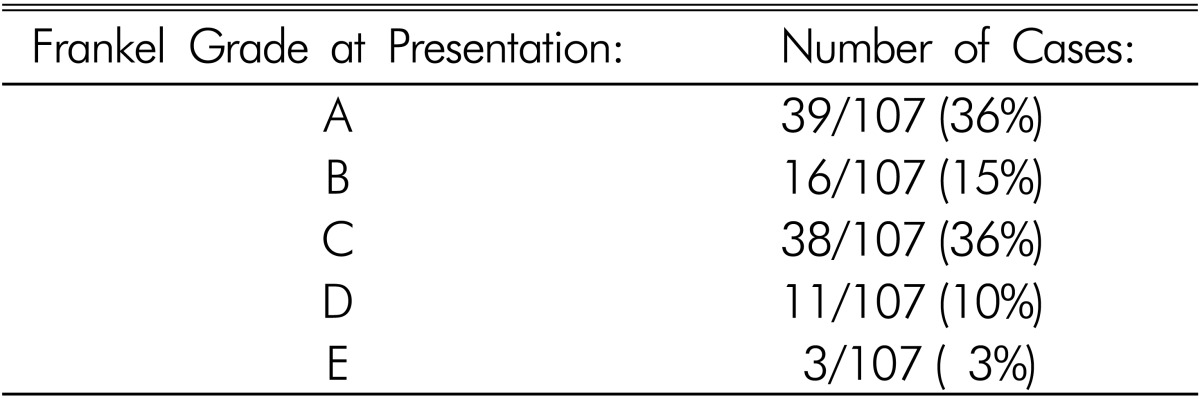
We identified 104 cases of SSEH in the literature and added our three patients for a total of 107 cases (Table 2). Nineteen patients had no significant past medical history. Fourteen patients had a history of anticoagulation (including use of Aspirin and Ibuprofen) and thirteen had a history of hypertension and five patients had hemophilia. Other past medical histories of significance included previous stroke, pregnancy, amyloidosis, renal transplant, epilepsy, and a prior history of SSEH. In thirteen patients the past medical history was not presented. The neurological deficits varied in severity from minor sensory abnormalities to complete paralysis. In four patients, severe neck pain or headache was the only presenting symptom. The majority of patients (93/107) presented with neurological impairment leading to loss of function (Table 3).
Table 2. Demographics of SSEH patients.
Table 3. Number of patients who presented with varying degrees of neurological dysfunction, as described by the Frankel grade.
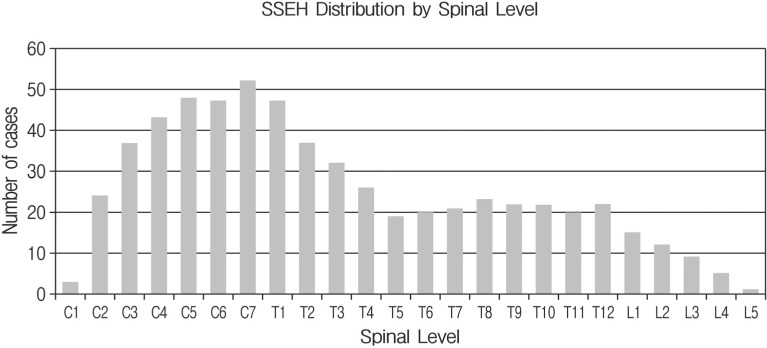
In 98 cases, MRI was used to make the diagnosis of SSEH. A CT scan was performed in five and a CT myelogram was performed in three of the cases. A conventional myelogram was used in one case. The average number of spinal segments involved was 5.4 with a range of 1 to 23. The distribution of segments showed increased involvement at the cervicothoracic junction (Fig. 6). The majority (53%) of hematomas were located posterior to the spinal cord, whereas 23% were located posterolateral, 18% were anterior, 5% were lateral and 1% were circumferential to the cord.
Fig. 6. Distribution of spinal segments involved in all reviewed cases of SSEH. There is increased frequency in the cervico-thoracic and to a less degree, the thoraco-lumbar regions. Overall there is increased involvement of the cervical and upper thoracic regions.
Overall, 60/107 (56%) of the patients had complete recovery. Of the 80 operative cases, 35 (43.75%) experienced complete recovery, 22 (27.5%) had incomplete motor recovery with good functional outcome, 10 (12.5%) had incomplete motor recovery with poor functional outcome, 2 (2.5%) had preserved sensation without motor function and 11 (13.75%) had no motor or sensory recovery. No patients suffered from worse neurological outcome after treatment.
For the statistical analysis investigating the effect of surgical timings on patient outcome, 43 of the 107 patients were excluded from the analysis for the following reasons: twenty-three patients were managed conservatively and thus would not provide any information on the effect of delayed surgery. We also excluded nine infants (<2 years old) from the analysis due to considerable delays in diagnosis, and consequently, substantially late presentation to the OR. As a result, age and time of surgery would be confounded. Finally, for six patients, missing data precluded analysis.
Of the 64 patients analyzed, 31 (48%) were female and 33 (52%) were male. The mean age of the patients was 48 (±24) with the following distribution among the four age categories: less than 20 years (n=9), 20 to 40 years (n=16), 40 to 60 years (n=12) and over 60 years (n=27). Forty-nine patients had surgery within 24 hours and 19 patients had surgery after 24 hours. The number of patients with an initial Frankel grade of A, B, C and D were 28, 9, 22 and 5, respectively.
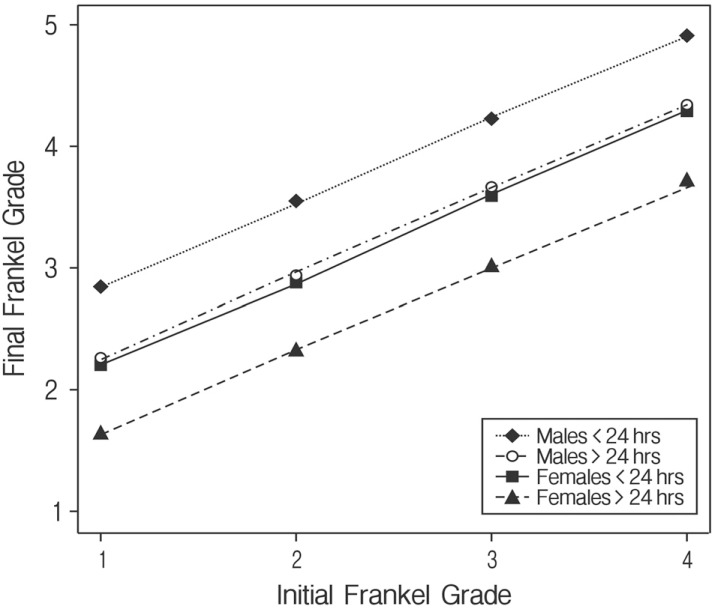
Initial Frankel grade (F(1, 60)=23.15, p=0.001) and sex (F(1, 60)=4.41, p=0.040) were statistically significant to final Frankel grade. For every one grade increase in the initial grade, the final grade increased by 0.69. After controlling for initial Frankel grade, male patients had better outcomes from surgery than female patients. The final Frankel grade of male patients averaged 0.63 points higher than female patients after adjusting for initial Frankel grade and time to surgery.
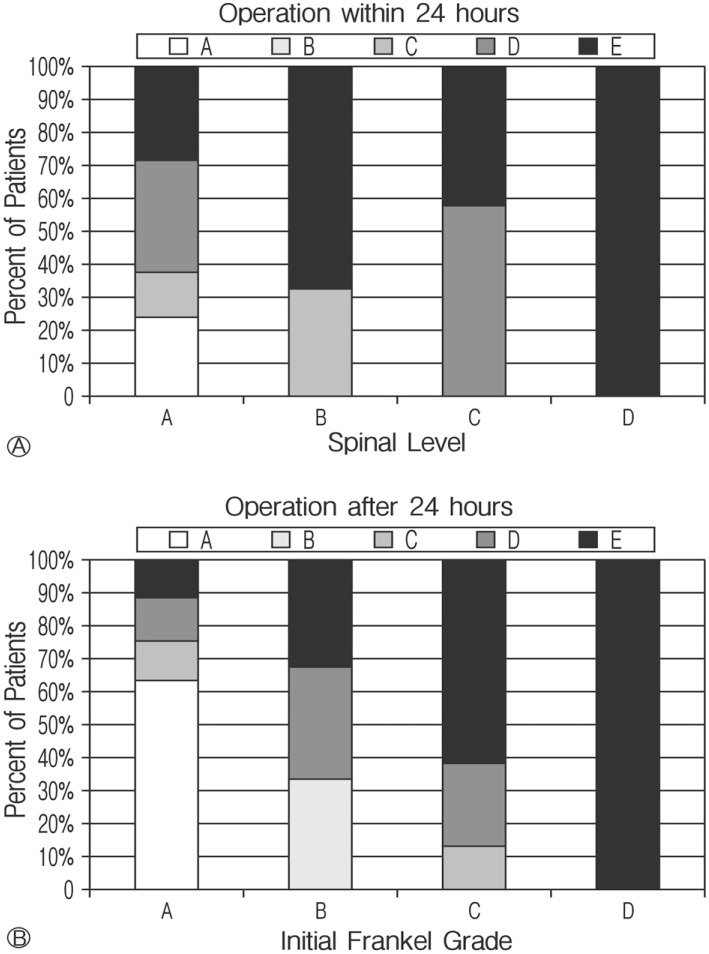
Although the timing of the surgery after admission was not statistically significant (F(1, 60)=3.18, p=0.079) to final Frankel grade, early surgery (within 24 hours) yielded better outcomes than delayed surgery (after 24 hours) (Fig. 7). On average, patients who received surgery within 24 hours had a final Frankel grade 0.60 points higher than those receiving later surgery (Fig. 8).
Fig. 7. Approximately 62% patients with an initial Frankel grade of A that had surgery after 24 hours do not improve. In contrast, only about 24% of Initial A; grade patients operated within 24 hours failed to improve. All patients with an initial Frankel grade of B; improved when operated on within 24 hours while 33% of those operated on after 24 hours remained without motor function.
Fig. 8. Initial and final Frankel grades.
On the other hand, age (F(3, 57)=0.96, p=0.42), past history of hypertension (F(1,57)=0.0013, p=0.97), use of anticoagulants (F(1, 57)=0.57, p=0.45), and the number of spinal segments in the hematoma (F(1, 57)=0.99, p=0.32) were not significant predictors of final Frankel grade. In addition, no two-way interactions were significant.
DISCUSSION
The first reported case of SSEH was by Jackson in 1869. Since then, over 400 cases have been described in the world literature. MRI has emerged as the diagnostic study of choice for SSEH. A T2-weighted scan showing an epidural mass hyperintense to the spinal cord with focal areas of hypointensity (consistent with the presence of deoxyhemoglobin) highly suggests the presence of an epidural hematoma26,27). No MRI characteristic that may predict outcome currently exists27).
In the majority of reports, the hematoma is considered to be "spontaneous" if it develops without known cause (i.e. trauma, fracture, spinal lesion or medical procedure). Hence, some refer to SSEH as idiopathic spinal epidural hematoma41). In most cases, however, patients have some history (i.e. anticoagulation use, bleeding diathesis, hypertension, straining) that may contribute to the bleeding. Some case reports include SSEH due to vascular malformations, whereas most authors do not consider these to be spontaneous in origin. Following a variable time interval anywhere from minutes to weeks, neurological dysfunction develops ranging from mild weakness to complete paralysis. Past reviews found that better prognosis is associated with less severe initial neurological dysfunction and with faster time to operation30,36,41,50). However, reported cases of SSEH managed non-surgically with steroids21) or only observation also exist17).
Past reviews found that pediatric patients presented with longer and more variable times to neurological symptom onset17). We believe this is due to difficulty in assessing pain and performing accurate neurologic examination in this group. Children under two years of age presented to surgeons after days, and sometimes weeks after symptoms eventually alerted parents or caregivers. Such symptoms included chronic irritability, crying and inability to ambulate or stand. Elderly patients, on the other hand, had faster onset to deficits varying from minutes to hours.
Treatment consisted of either decompressive laminectomy or conservative management with or without steroids. In our study, conservative management was employed in 23 patients. In one of these, neurological improvement occurred following a lumbar puncture (LP), which drew 20ml of clear cerebrospinal fluid (CSF)3). In this case, the LP was performed to rule out infection before MRI diagnosis of the epidural hematoma. The remaining 84 patients were treated surgically. Of these, 47 were operated within 24 hours of symptom onset and 37 were operated 24 hours after symptom onset.
1. Prognostic Factors
For those cases treated surgically, the literature reports that post-operative recovery is based both on pre-operative neurological function as well as time to operation after the onset of these symptoms30,41,50). Our review also illustrates that patients with a higher pre-operative Frankel grade, functional recovery was more likely. Also, those patients with a shorter interval to operation had better recovery than those with longer intervals. Especially in cases of complete neurological dysfunction, a timely operation may be of great benefit, and even in cases of delayed surgery, good functional outcome can be achieved. In contrast to Groen's review and for unknown reason, recovery was greater in males than in females after surgery.
A past medical history that may have contributed to bleeding is associated with a worse neurological presentation and may indirectly influence eventual recovery. In 10 of the 26 cases of complete initial motor deficit, patients had a history of anticoagulation such as aspirin or ibuprofen use.
However, even patients with significant comorbidities did achieve functional recovery comparable with patients without comorbidities. Hence, a relevant past medical history does not predict poor outcome, but may be associated with worse initial neurological dysfunction than others.
Initial neurological dysfunction was the strongest predictor for a patient's outcome. Patients presenting with severe and/or worsening conditions typically did not recover as well as those presenting with minor symptoms. Furthermore, the timing of a decompressive laminectomy was more significant factor in patients initially presenting with Frankel grades of A or B. Patients presenting with better neurologic function (scores of D or E) frequently showed substantial improvement regardless of time to operation. With the exception of patients showing significant neurologic improvement before surgery, a prompt evacuation of an epidural hematoma should be attempted. Even in the case of complete or severe neurologic deficit and lengthy symptom duration, functional recovery may be achieved through surgical intervention.
2. Pathophysiology
Groen speculated that bleeding most likely arises from the posterior epidural venous plexus33). This plexus lies directly between the dura and ligamentum flavum making rupture more likely than in the anterior venous plexus which is covered by the posterior longitudinal ligament. Communication between the venous plexus and the intrathoracic veins allows for transfer of pressures from one compartment to another. Given that the epidural venous plexus is devoid of valves, it may be particularly vulnerable to sudden changes in pressure, especially at the cervicothoracic and thoracolumbar junctions where resistance is minimal. Cases of SSEH associated with heavy lifting, straining, pregnancy and hypertension further supports the venous theory.
In contrast, Beatty suggested that bleeding occurs from epidural arteries16). He argued that hemorrhage from an epidural vein would not create enough pressure to compress the spinal cord, observing that the normally expanded epidural sac may tamponade epidural bleeding encountered during surgical procedures. There are also reports of SSEH located anteriorly for which the posterior venous plexus theory would not apply4,5,11,12,19,20,24,26,28,29,34,37,44,51). With few exceptions, SSEH arises from a venous bleed rather than an arterial bleed.
3. Treatment
In recent years, there have been increased reports of spontaneously resolving spinal epidural hematomas. This may be due to quick diagnosis by higher resolution MRI when a patient presents with sudden onset of neurological deficits. Even in the face of improving neurological condition, MRI is performed to evaluate the size and location of the hematoma32). It is important to stress that by the time of diagnosis with MRI, the patients managed conservatively were already improving clinically. Thus, conservative management is only appropriate in the face of improving neurological status.
Four of the five hemophiliac patients in our review were treated conservatively with either Factor VIII or Factor IX? both of which are clotting-related proteins. All four of these patients recovered to an "E" Frankel grade. Although the number of hemophiliac patients is relatively small, there has been sub stantial success of conservative management in these patients.
In a review by Groen, conservatively treated SSEH had more spinal levels involved than those treated operatively. Similarly in our review, the mean spinal levels of SSEH managed conservatively was seven as opposed to five for surgical cases. The total length of involvement was not a dependent factor predicting outcome.
CONCLUSION
Both the venous and arterial theories provide plausible explanations for SSEH with most cases probably resulting from a venous bleed. Our review of SSEH presented in the English literature since 1996 is consistent with previously published reviews with regard to clinical presentation, spinal segment involvement and factors predicting neurological outcome except males as a group did better than females for unknown reason. Patients with less initial neurological deficit and those with shorter time to operation show better neurological improvement. Those who present with a past medical history are more likely to have greater initial neurological deficit with tendency to greater bleeding, especially in the case of anticoagulation use. An increasing number of conservatively treated SSEH cases have been reported. In all such cases, patients presented with improving neurological status. Although high resolution MRI has improved the accuracy of SSEH diagnosis, no imaging characteristics serve to predict the eventual outcome. Expedient decompressive laminectomy continues to be the treatment of choice for patients with SSEH.
Acknowledgments
We would like to acknowledge Sandra Taylor, PhD for her guidance and feedback in statistical analysis. Statistical support was made possible by grant # UL1 RR024146 from the National Center for Research Resources (NCRR), a component of the National Institute of Health (NIH) and NIH Roadmap for Medical Research. The contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
References
- 1.Abram HS, DeLaHunt MJ, Merinbaum DJ, Hammond DN. Recurrent spontaneous spinal epidural hematoma in a child: first case report. Pediatr Neurol. 2007;36(3):177–180. doi: 10.1016/j.pediatrneurol.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Alexiadou-Rudolf C, Ernestus RI, Nanassis K, Lanfermann H, Klug N. Acute nontraumatic spinal epidural hematomas. An important differential diagnosis in spinal emergencies. Spine. 1998;23(16):1810–1813. doi: 10.1097/00007632-199808150-00018. [DOI] [PubMed] [Google Scholar]
- 3.Alexiadou-Rudolf C, Ernestus RI, Nanassis K, Lanfermann H, Klug N. Acute nontraumatic spinal epidural hematomas. An important differential diagnosis in spinal emergencies. Spine. 1998;23(16):1810–1813. doi: 10.1097/00007632-199808150-00018. [DOI] [PubMed] [Google Scholar]
- 4.Aoki Y, Yamagata M, Shimizu K, Ikeda Y, Nakajima F, Ohtori S, et al. An unusually rapid spontaneous recovery in a patient with spinal epidural hematoma. J Emerg Med. 2009 doi: 10.1016/j.jemermed.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 5.Awada A, Russell N, al Fayez N, Naufal R, al Kohlani H. Spontaneous cervical epidural hematoma: case report. Spinal Cord. 1998;36:71–72. doi: 10.1038/sj.sc.3100521. [DOI] [PubMed] [Google Scholar]
- 6.Badar F, Kirmani S, Rashid M, Azfar SF, Yasmeen S, Ullah E. Spontaneous spinal epidural hematoma during pregnancy: a rare obstetric emergency. Emerg Radiol. 2011;18(5):433–436. doi: 10.1007/s10140-011-0952-9. [DOI] [PubMed] [Google Scholar]
- 7.Baek BS, Hur JW, Kwon KY, Lee HK. Spontaneous spinal epidural hematoma. J Korean Neurosurg Soc. 2008;44(1):40–42. doi: 10.3340/jkns.2008.44.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bisson EF, Dumont T, Tranmer B. Spontaneous spinal epidural hematoma in a child with hemophilia B. Can J Neurol Sci. 2007;34(4):488–490. doi: 10.1017/s0317167100007423. [DOI] [PubMed] [Google Scholar]
- 9.Bose S, Ali Z, Rath GP, Prabhakar H. Spontaneous spinal epidural haematoma: a rare cause of quadriplegia in the post-partum period. Br J Anaesth. 2007;99(6):855–857. doi: 10.1093/bja/aem265. [DOI] [PubMed] [Google Scholar]
- 10.Carroll SG, Malhotra R, Eustace D, Sharr M, Morcos S. Spontaneous spinal extradural hematoma during pregnancy. J Matern Fetal Med. 1997;6:218–219. doi: 10.1002/(SICI)1520-6661(199707/08)6:4<218::AID-MFM6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 11.Chang MO, Koh ES, Kim MJ, Chang YS, Chung S. Spontaneous spinal epidural hematoma. QJM. 2012;105(7):705–706. doi: 10.1093/qjmed/hcr097. [DOI] [PubMed] [Google Scholar]
- 12.Chen CJ, Fang W, Chen CM, Wan YL. Spontaneous spinal epidural hematomas with repeated remission and relapse. Neuroradiology. 1997;39:737–740. doi: 10.1007/s002340050498. [DOI] [PubMed] [Google Scholar]
- 13.Chen CL, Lu CH, Chen NF. Spontaneous spinal epidural hematoma presenting with quadriplegia after sit-ups exercise. Am J Emerg Med. 2009;27(9):1170 e3–1170 e7. doi: 10.1016/j.ajem.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Cirak B, Guven MB, Akalan N. Spontaneous spinal epidural hematoma. Arch Phys Med Rehabil. 1999;80(1):125. doi: 10.1016/s0003-9993(99)90321-0. [DOI] [PubMed] [Google Scholar]
- 15.Cywinski JB, Parker BM, Lozada LJ. Spontaneous spinal epidural hematoma in a pregnant patient. J Clin Anesth. 2004;16:371–375. doi: 10.1016/j.jclinane.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 16.David S, Salluzzo RF, Bartfield JM, Dickinson ET. Spontaneous cervicothoracic epidural hematoma following prolonged valsalva secondary to trumpet playing. Am J Emerg Med. 1997;15:73–75. doi: 10.1016/s0735-6757(97)90054-1. [DOI] [PubMed] [Google Scholar]
- 17.Deger SM, Emmez H, Bahadirli K, Kale A, Ebinc FA, Turkoglu M, et al. A spontaneous spinal epidural hematoma in a hemodialysis patient: a rare entity. Intern Med. 2009;48(24):2115–2118. doi: 10.2169/internalmedicine.48.2335. [DOI] [PubMed] [Google Scholar]
- 18.Duffill J, Sparrow OC, Millar J, Barker CS. Can spontanoeous spinal epidural hematoma be managed safely without operation? a report of four cases. J Neurol Neurosurg Psychiatry. 2000;69:816–819. doi: 10.1136/jnnp.69.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forsnes E, Occhino A, Acosta R. Spontaneous spinal epidural hematoma in pregnancy associated with using low molecular weight heparin. Obstet Gynecol. 2009;113:532–533. doi: 10.1097/AOG.0b013e31818f52d1. [DOI] [PubMed] [Google Scholar]
- 20.Fukui MB, Swarnkar AS, Williams RL. Acute spontaneous spinal epidural hematomas. AJNR. 1999;20:1365–1372. [PMC free article] [PubMed] [Google Scholar]
- 21.Gaffney P, Guthrie JA. Spontaneous spinal epidural haematoma: an unusual cause of neck pain. J Accid Emerg Med. 2000;17:229–232. doi: 10.1136/emj.17.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Groen RJ, van Alphen HA. Operative treatment of spontaneous spinal epidural hematomas: A study of the factors predicting postoperative outcome. Neurosurgery. 1996;39(3):494–509. doi: 10.1097/00006123-199609000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Groen RJ. Non-operative treatment of spontaneous spinal epidural hematomas: a review of the literature and a comparison with operative cases. Acta Neurochir (Wien) 2004;146:103–110. doi: 10.1007/s00701-003-0160-9. [DOI] [PubMed] [Google Scholar]
- 24.Halim TA, Nigam V, Tandon V, Chhabra HS. Spontaneous cervical epidural hematoma: report of a case managed conservatively. Indian J Orthop. 2008;42(3):357–359. doi: 10.4103/0019-5413.41863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helmy A, Mellor G. Spontaneous cervical cord haemorrhage: an unusual presentation. Emerg Med J. 2007;24(3):e16. doi: 10.1136/emj.2006.042804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hentschel SJ, Woolfenden AR, Fairholm DJ. Resolution of spontaneous spinal epidural hematoma without surgery: report of two cases. Spine. 2001;26(22):E525–E527. doi: 10.1097/00007632-200111150-00025. [DOI] [PubMed] [Google Scholar]
- 27.Hsieh CT, Chang CF, Lin EY, Tsai TH, Chiang YH, Ju DT. Spontaneous spinal epidural hematomas of cervical spine: report of 4 cases and literature review. Am J Emerg Med. 2005;24(6):736–740. doi: 10.1016/j.ajem.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 28.Inamasu J, Hori S, Aoki K, Aikawa N, Maruiwa H, Toyama Y. Spontaneous spinal epidural hematoma. Am J Emerg Med. 2000;18(7):837–839. doi: 10.1053/ajem.2000.18081. [DOI] [PubMed] [Google Scholar]
- 29.Jea A, Moza K, Levi AD, Vanni S. Spontaneous spinal epidural hematoma during pregnancy: case report and literature review. Neurosurgery. 2005;56(5):E1156. [PubMed] [Google Scholar]
- 30.Kiehna EN, Waldron PE, Jane JA. Conservative management of an acute spontaneous holocord epidural hemorrhage in a hemophiliac infant. J Neurosurg Pediatr. 2010;6(1):43–48. doi: 10.3171/2010.4.PEDS09537. [DOI] [PubMed] [Google Scholar]
- 31.Kiehna EN, Waldron PE, Jane JA. Conservative management of an acute spontaneous holocord epidural hemorrhage in a hemophiliac infant. J Neurosurg Pediatr. 2010;6(1):43–48. doi: 10.3171/2010.4.PEDS09537. [DOI] [PubMed] [Google Scholar]
- 32.Kirazli Y, Akkoc Y, Kanyilmaz S. Spinal epidural hematoma associated with oral anticoagulation therapy. Am J Phys Med Rehabil. 2004;83(3):220–223. doi: 10.1097/01.phm.0000107498.91919.44. [DOI] [PubMed] [Google Scholar]
- 33.Kong J, Mak KH. Spontaneous spinal epidural haematoma - An unusual cause of spinal cord compression. Hong Kong Med J. 2003;9(1):55–57. [PubMed] [Google Scholar]
- 34.Kotilainen EM, Pajulo O. Spontaneous epidural hematoma as a cause of sciatic pain in a schoolboy. Pediatr Neurol. 1997;17(4):350–352. doi: 10.1016/s0887-8994(97)00095-7. [DOI] [PubMed] [Google Scholar]
- 35.Lawton MT, Porter RW, Heiserman JE, Jacobowitz R, Sonntag VK, Dickman CA. Surgical management of spinal epidural hematoma: relationship between timing and neurological outcome. J Neurosurg. 1995;83(1):1–7. doi: 10.3171/jns.1995.83.1.0001. [DOI] [PubMed] [Google Scholar]
- 36.Liu WH, Hsieh CT, Chiang YH, Chen GJ. Spontaneous spinal epidural hematoma of thoracic spine: a rare case report and review of literature. Am J Emerg Med. 2008;26(3):384.e1–384.e2. doi: 10.1016/j.ajem.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 37.Lonjon MM, Paquis P, Chanalet S, Grellier P. Nontraumatic spinal epidural hematoma: report of four cases and review of the literature. Neurosurgery. 1997;41(2):483–487. doi: 10.1097/00006123-199708000-00035. [DOI] [PubMed] [Google Scholar]
- 38.Meena AK, Jayalakshmi S, Prasad VS, Murthy JM. Spinal epidural haematoma in a patient with haemophilia-B. Spinal Cord. 1998;36:658–660. doi: 10.1038/sj.sc.3100666. [DOI] [PubMed] [Google Scholar]
- 39.Miller JB, Khalsa G, Vohra T. Spontaneous spinal epidural hematoma presenting as flank pain and constipation. Am J Emerg Med. 2010;28(4):536 e3–536 e5. doi: 10.1016/j.ajem.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 40.Morales Ciancio RA, Drain O, Rillardon L, Guigui P. Acute spontaneous spinal epidural hematoma: an important differential diagnosis in patients under clopidogrel therapy. Spine J. 2008;8(3):544–547. doi: 10.1016/j.spinee.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 41.Muthukumar N. Chronic spontaneous spinal epidural hematoma - a rare cause of cervical myelopathy. Eur Spine J. 2003;12:100–103. doi: 10.1007/s00586-002-0404-z. [DOI] [PubMed] [Google Scholar]
- 42.Nourbakhsh A, Chaljub G, Garges KJ. Spontaneous cervical epidural hematoma masquerading as an abscess on magnetic resonance imaging scan. J Manipulative Physiol Ther. 2009;32(5):391–395. doi: 10.1016/j.jmpt.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 43.Oh JY, Lingaraj K, Rahmat R. Spontaneous spinal epidural haematoma associated with aspirin intake. Singapore Med J. 2008;49(12):e353–e355. [PubMed] [Google Scholar]
- 44.Pahapill PA, Lownie SP. Conservative treatment of acute spontaneous spinal epidural hematoma. Can J Neurol Sci. 1998;25:159–163. doi: 10.1017/s0317167100033795. [DOI] [PubMed] [Google Scholar]
- 45.Pai SB, Maiya PP. Spontaneous spinal epidural hematoma in a toddler - a case report. Childs Nerv Syst. 2006;22(5):526–529. doi: 10.1007/s00381-005-0002-6. [DOI] [PubMed] [Google Scholar]
- 46.Poonai N, Rieder MJ, Ranger A. Spontaneous spinal epidural hematoma in an 11-month-old girl. Pediatr Neurosurg. 2007;43(2):121–124. doi: 10.1159/000098385. [DOI] [PubMed] [Google Scholar]
- 47.Ramelli GP, Boscherini D, Kehrli P, Rilliet B. Spontaneous spinal epidural hematomas in children: can we prevent a negative prognosis? - - reflections on 2 cases. J Child Neurol. 2008;23(5):564–567. doi: 10.1177/0883073807309777. [DOI] [PubMed] [Google Scholar]
- 48.Rohde V, Kker W, Reinges MH, Gilsbach JM. Microsurgical treatment of spontaneous and non-spontaneous spinal epidural haematomas: neurological outcome in retalion of aetiology. Acta Neurochir (Wien) 2000;142:787–792. doi: 10.1007/s007010070093. discussion 792-793. [DOI] [PubMed] [Google Scholar]
- 49.Rosenberg O, Itshayek E, Israel Z. Sontaneous spinal epidural hematoma in a 14 year old girl. Pediatr Neurosurg. 2003;38:216–218. doi: 10.1159/000069091. [DOI] [PubMed] [Google Scholar]
- 50.Sakakibara R, Yamazaki M, Mannouji C, Yamaguchi C, Uchiyama T, Ito T, et al. Urinary retention without tetraparesis as a sequel to spontaneous spinal epidural hematoma. Intern Med. 2008;47(7):655–657. doi: 10.2169/internalmedicine.47.0765. [DOI] [PubMed] [Google Scholar]
- 51.Sirin S, Arslan E, Yasar S, Kahraman S. Is spontaneous spinal epidural hematoma in elderly patients an emergency surgical case? Turk Neurosurg. 2010;20(4):557–560. doi: 10.5137/1019-5149.JTN.2338-09.1. [DOI] [PubMed] [Google Scholar]
- 52.Song KJ, Lee KB. The poor ouctome of the delayed diagnosis of acute spontaneous spinal epidural hematoma: two cases report. J Korean Med Sci. 2005;20:331–334. doi: 10.3346/jkms.2005.20.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Schaeybroeck P, Van Calenbergh F, Van De Werf F, Demaerel P, Goffin J, Plets C. Spontaneous spinal epidural hematoma associated with thrombolysis and anticoagulation therapy: report of three cases. Clin Neurol Neurosurg. 1998;100:283–287. doi: 10.1016/s0303-8467(98)00044-4. [DOI] [PubMed] [Google Scholar]
- 54.Wagner S, Forsting M, Hacke W. Spontaneous resolution of a large spinal epidural hematoma: case report. Neurosurgery. 1996;38:816–818. [PubMed] [Google Scholar]
- 55.Woon CY, Peng BC, Chen JL. Spontaneous spinal epidural haematomas and the prognostic implications of interval to surgical decompression: a report of two cases. J Orthop Surg (Hong Kong) 2009;17(2):216–219. doi: 10.1177/230949900901700220. [DOI] [PubMed] [Google Scholar]