Figure 4.
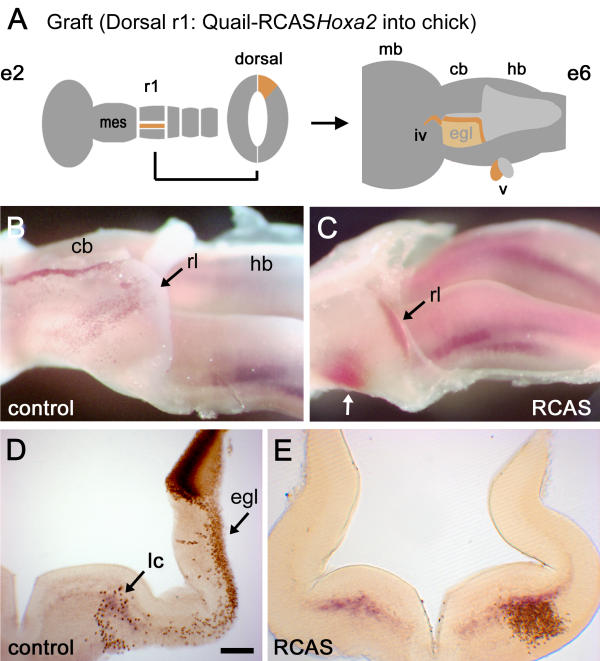
Targeted Hoxa2 overexpression respecifies the migration of rhombic lip derivatives (A) Schematic diagram showing the location of donor tissue grafted from Hoxa2 electroporated quail embryos at embryonic day (E) 2 (e2; left). A graft of dorsal rhombomere 1 (r1) maps to the cerebellar rhombic lip and roofplate at E6 (e6; right) and contributes to both the external granule cell layer (EGL) and neural crest derivatives in the trochlear (iv) and trigeminal (v) nerves [2]. (B) Lateral view of a wholemount dissection of the cerebellum (cb) and hindbrain (hb) of a control E6 chimaeric embryo stained with a quail-specific antibody, Q¢PN (brown), and stained for Phox2a (purple). The rostrocaudal axis runs left to right. A stream of ventrally migrating quail cells originating from caudal rhombic lip (rl) lies over the presumptive cerebellum (point of origin indicated by a black arrow). A thin strip of quail cells also lies in the roofplate separating the bilateral anlage. (C) Migration patterns are substantially altered when grafted donor cells (black arrow) are electroporated with RCASBP(B)Hoxa2. Rhombic lip derivatives fail to condense in the EGL but rather migrate into a single ventrolateral nucleus (white arrow), which expresses high levels of ectopic Hoxa2 (red). (D) Transverse section through the cerebellum of a control chimaera showing the donor progenitors in the dorsal neural tube. The majority of quail cell derivatives lie within the EGL (egl), with a scattering of cells ventrally overlapping the Phoxa2 expression in the locus coeruleus (light blue). (E) By contrast, the majority of cells from grafts electroporated with Hoxa2 condense ventrally (where quail cell nuclear diaminobenzidine label obscures the red in situ product marking Hoxa2). Scale bar (D,E) = 200 μm. mb – midbrain; mes – mesencephalon.
