Abstract
Purpose
To develop an MR-compatible resistive heater for temperature maintenance of anaesthetized animals.
Materials and Methods
An MR-compatible resistive electrical heater was formed from a tightly-wound twisted pair wire, interfaced to a homeothermic maintenance controller. Fat-suppressed images and localized spectra were acquired with the twisted pair heater and a near-identical single strand heater during operation at maximum power. Data were also acquired in the absence of heating to demonstrate the insensitivity of MR to distortions arising from the passage of current through the heater elements. The efficacy of temperature maintenance was examined by measuring rectal temperature immediately following induction of general anesthesia and throughout and after the acquisition of a heater artifact-prone image series.
Results
Images and spectra acquired in the presence and absence of DC current through the twisted pair heater were identical whereas the passage of current through the single strand wire created field shifts and lineshape distortions. Temperature that is lost during anesthesia induction was recovered within approximately 10–20 minutes of induction, and a stable temperature is reached as the animal's temperature approaches the set target.
Conclusion
The twisted pair wire heater does not interfere with MR image quality and maintains adequate thermal input to the animal to maintain body temperature.
Keywords: Thermoregulation, B0-insensitive heating, Small animal imaging
1. Introduction
General anesthesia is necessary for subject immobilization in most preclinical imaging studies but causes unavoidable alterations in body function including the loss of thermoregulation. Anesthesia-induced hypothermia has several negative consequences for the animal, altering a multitude of physiological processes, prolonging recovery times post anesthesia, reducing resistance to infection or, in the worst case, resulting in death [1–3]. The loss of temperature occurs very easily in small rodents due to their high body surface area to volume ratio, and their reliance upon locomotor activity for heat generation [4,5]. In addition, substantial body heat is lost due to exposure to actively-cooled procedure rooms and apparatus and the delivery of room temperature anesthetic carrier gases. Active heating of the animal is, therefore, required to prevent hypothermia when anesthetic is used. Several methods for providing heat have been described including the use of heat lamps, circulating warm liquids or air, pre-warmed heat reservoirs and exothermic chemical reactions [6–9]. Although any of these devices can provide thermal support, many of them are not appropriate for use in preclinical MRI scanners as they are either too big or they distort the images. Circulating liquids require space which may not be available when optimal RF coil loading and signal-to-noise ratios for high sensitivity MRI are required. In addition, circulating air heats the RF coils of which the performance is heat-sensitive. Heat lamps require a line-of-sight to the animal, and exothermic reaction devices require space and intimate contact with the animal, so these methods may not be practicable. Electrical systems can operate with a much-reduced volume requirement but have the potential to disturb the magnetic field and distort the images [10].
Here, we present an electrical heating system using a narrow diameter twisted pair resistor wire, built into the animal loading cradle, that presents no detectable image artifacts, and which is suitable for use within a small volume coil at high magnetic field where the effective use of circulating warm air or liquids, the standard methods of temperature maintenance in preclinical MRI, is not possible.
2. Materials and methods
2.1. Ethics statement
All animal studies were performed in full compliance with national legislation and with the approval of the local animal ethics committee.
2.2. Animal preparation
Female CBA and Balb/c Nude mice (Charles-River) were housed in individual ventilated cages at constant temperature and humidity, and water and food were freely available. CBA mice were used to measure the core body temperature of the awake mouse while the former were used to demonstrate efficacy of temperature maintenance using the resistive heater. All mice were placed in a head-first prone position for imaging. Anesthesia was induced and maintained using isoflurane (1–4%) in air, and animals were recovered afterwards with no ill effect. Core body temperature was measured using a rectal temperature probe interfaced with a homeothermic temperature maintenance system (Harvard Apparatus, model: 507221 F). Depth of anesthesia was monitored using a pressure balloon system measuring the animal's respiration rate, which was maintained at 60–90 breaths/minute. ECG signals were monitored using needles positioned subcutaneously in the chest (400–450 heart beats/minute). Both ECG and respiratory signals were processed for use in gating control of the scanner to allow for cardio-respiratory synchronized DCE-MRI [11].
2.3. MR imaging
MR was performed on a 4.7 T, 310 mm VNMRS horizontal bore preclinical imaging system equipped with a 120 mm bore gradient insert capable of 400 mT/m, slew rate 3077 mT/m/ms, and a duty cycle of 12.5% at maximum gradient strength simultaneously in all three axes (Varian, Inc., Palo Alto, CA). RF transmission and reception was performed with a 25 mm ID quadrature birdcage coil with RF window length 35 mm (Rapid Biomedical GmbH, Rimpar, Germany).
Fat-suppressed respiration gated gradient echo imaging (TR 100 ms, TE 2.15 ms, FA 30°, THK 1 mm, image matrix 128 × 128 and in-plane resolution 200 μm), and PRESS localized spectroscopy (TE 13.7 ms, NEX 1) was performed to highlight any image distortions arising from the resistive heater. A DCE-MRI type scan was performed during homeothermic maintenance monitoring using an RF and gradient-spoiled 3D gradient echo sequence (cardio-respiratory gated, TR 1.15 ms, TE 0.6 ms, FA 5°, image matrix 128 × 64 × 64 and 422 μm isotropic resolution) to demonstrate temperature maintenance, even when the scanner acts as a significant thermal input to the mouse.
2.4. Resistive heater
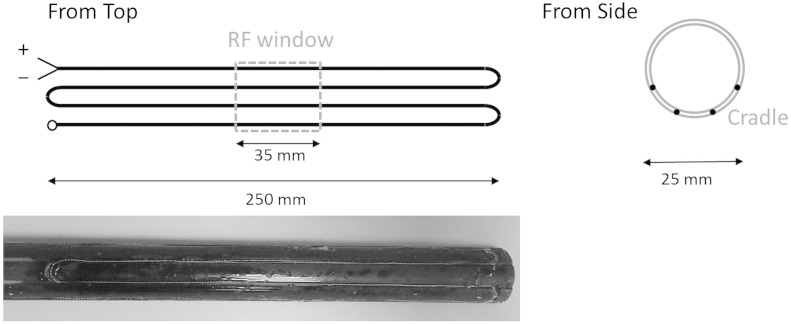
A commercially available homeothermic temperature maintenance system was modified by replacing the standard heater pad with a 2 Ω resistor, generating a 2 W loop, and a 10 Ω power resistor, both placed in series. The former was made from a 150 μm diameter copper wire that was formed into a tightly wound (12 turns/cm) twisted pair which was embedded into the fiberglass animal loading cradle in the form of four parallel lanes, schematically shown in Fig. 1, without increasing the cradle's size.
Fig. 1.
Schematic and photograph of the heater wire and cradle apparatus.
The lanes of the resistor pass axially through the RF coil and are sufficient in length to cover the length of the animal. The twisted pair was designed to minimize any B0 field distortions that would arise from passing DC current through a single strand wire. The power resistor was positioned remotely from the animal and magnet and limited the loop current to a maximum of approximately 1 A. A rectal thermocouple was used to provide feedback to the homeothermic controller unit. The temperature distribution of the upper surface of the empty cradle was assessed by both thermal imaging (Testo thermal imaging cameral, model 875–1) and use of the rectal thermocouple when the homeothermic controller unit was set to 37 °C.
2.5. MR-compatibility
Demonstration of insensitivity of the MRI experiment to the use of the twisted pair wire was performed with fat-suppressed gradient echo MRI and localized spectroscopy: in the absence of any heating current; in the presence of heating current passed through the twisted pair loop; and in the presence of heating current passed through a near-equivalent single wire element.
2.6. Homeothermic maintenance in mice
The efficacy of temperature maintenance was confirmed in Balb/c nude mice. To provide information about core temperature of the awake mouse in its cage, rectal temperature in CBA mice was measured using a digital thermometer (ATP, DT-610-B) immediately post mortem following cervical dislocation (n = 5).
A different set of mice (n = 5) was anaesthetized and placed prone into the imaging cradle containing the twisted pair heater wire. The target temperature of the homeothermic temperature maintenance control unit was set sequentially to 34, 35, 36 and 37 °C. For each target temperature, the mouse's core body temperature was maintained for 15 minutes once steady state was reached. Additionally, with the homeothermic controller still set to 37 °C, a DCE-MRI type scan, which provides an additional input of heat to the animal, was started. The scan ran continuously until the mouse's core temperature stabilized. Subsequently, the DCE scan was stopped, and the temperature was monitored until a steady state was restored for 15 minutes.
Besides the stepwise increase of the mouse's core temperature to show the controllability of the homeothermic maintenance system, a more realistic test was performed in order to recover the animal's heat loss upon anesthetic induction as fast as possible. Mice were anaesthetized, tail vein cannulated and transferred to the imaging cradle. Needles for ECG monitoring were placed subcutaneously. The homeothermic controller was set to 37 °C and the mouse's core temperature was monitored without (n = 5) and with (n = 5) the additional heat of a DCE-MRI type scan. The latter was applied until target temperature was reached.
3. Results
3.1. Resistive heater
Embedding the heater in four parallel lanes into the lower surface of the fiberglass cradle resulted in a temperature distribution of 34.8–35.6 °C over the upper surface of the cradle when the homeothermic controller unit was set to 37 °C. The embedded heater did not occupy extra space. Satisfactory RF coil tuning and matching was possible, and calibrated RF pulse powers were unaffected by presence of the heater wire.
The 2 W loop power dissipation generated by the twisted pair was sufficient to warm an animal to any single target temperature in the range of 34–37 °C (Fig. 2). As the animal approached the target temperature, the current delivered through the resistor was reduced to avoid any overheating. The power resistor also limited the maximum temperature of the upper surface of the cradle to 36 °C, even when the temperature controller is functioning continually at its maximum output. As a result, thermal injuries are avoided even at indefinitely long maximum heating.
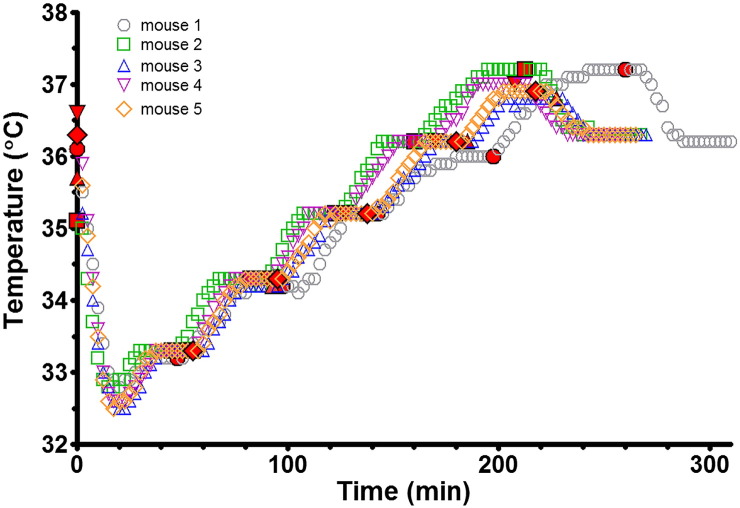
Fig. 2.
Efficacy of temperature maintenance. Temperature maintenance of 5 nude mice was evaluated using the twisted pair heater wire. The homeothermic controller unit was sequentially set to 34, 35, 36 and 37 °C after which a DCE-MRI type scan was applied and stopped. The red symbols indicate the time points at which the homeothermic controller was set to the next temperature or when the DCE-MRI type scan was initiated/stopped.
3.2. MR-compatibility
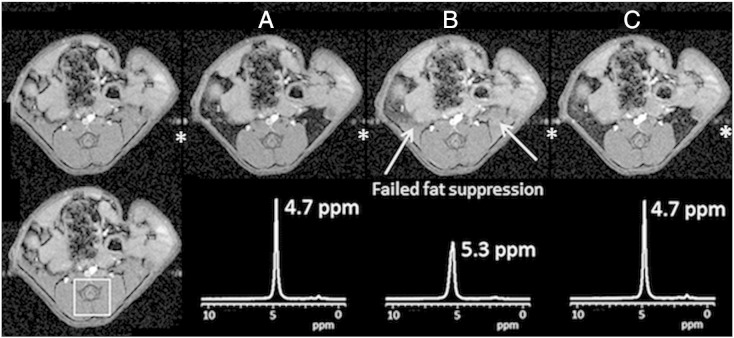
The use of the tightly wound twisted pair wire resulted in sufficient cancellation of induced magnetic fields arising from passage of current through the wire. Fig. 3 compares fat-suppressed imaging and localized spectroscopy performed in a mouse with different heating wire arrangements. Data were acquired with twisted pair heater wire and maximum current (A), single strand heater wire and maximum current (B), and without heater wire current (C). Temperature maintenance using the twisted pair heater wire traverses between states A and C in response to the temperature of the animal. Temperature maintenance using a traditional single strand heater wire traverses between states B and C.
Fig. 3.
MR-compatibility of the resistive heater. Fat-suppressed gradient echo imaging (top) and PRESS localized spectroscopy (bottom) performed with:
A. Twisted pair heater wire and maximum current.
B. Single strand heater wire and maximum current.
C. No heater wire current.
Top left. Reference image without fat suppression.
Bottom left. Voxel plan for localized spectroscopy.
⁎Residual motion artifacts due to absence of cardiac synchronization.
Although both twisted pair and single strand wiring arrangements were able to maintain the animal core temperature, significant differences in B0, line width and SNR were observed. The results in Fig. 3A demonstrate that B0 and SNR are essentially unaffected by passing variable DC current through the twisted pair heater for temperature maintenance. In particular, the water signal in the localized spectra of Fig. 3A and C differ in both linewidth and B0 by less than 1 Hz. As such, the effect on image and spectral quality is negligible. However, for the traditional single strand heater wire (Fig. 3B), the efficacy of fat suppression is compromised by spatially dependent induced B0 distortions which are apparent in the localized spectrum where the water peak is broadened from 33 Hz to 76 Hz and shifted by 120 Hz from 4.7 ppm to 5.3 ppm. These distortions, arising from current in the single strand heater wire, lead to field gradients and shifts, and a failure in fat suppression. However, as the images and spectra presented show, this is easily avoided by using the twisted pair heater. This set-up has allowed the use of mice weighing in excess of 30 g to be imaged within a 25 mm inner diameter coil.
3.3. Homeothermic maintenance in mice
Core mouse body temperature immediately following cervical dislocation was 36.9 °C ± 0.1 °C. A graph of the stepwise temperature increase of 5 anaesthetized nude mice using the twisted pair heater is shown in Fig. 2. The body temperature of the mice could be maintained at each chosen temperature. The DCE-MRI type scan increased the core temperature from 36.2 °C to 36.8–37.2 °C when the homeothermic controller unit was set to 37 °C. When the DCE-MRI type scan was stopped, the core temperature settled to 36.3 °C which is the steady-state temperature for the homethermic controller set to 37 °C.
The discrepancy between the requested and achieved temperature resulted from the use of a non-standard heater element; not that delivered with the system. As a consequence the standard, commercial homeothermic controller is not optimized. However, the ease with which the heater element could be deployed in conjunction with the otherwise unmodified homeothermic controller, and the consistency in the achieved temperature (− 0.7 to − 0.8 °C below target) meant that manual offset correction can be applied over the temperature range 34–37 °C. Heating elements have been produced for RF coils in the diameter range 25–72 mm, and equivalent performance to that demonstrated here has been achieved at 4.7, 7.0 and 9.4 T in rats and mice (data not shown).
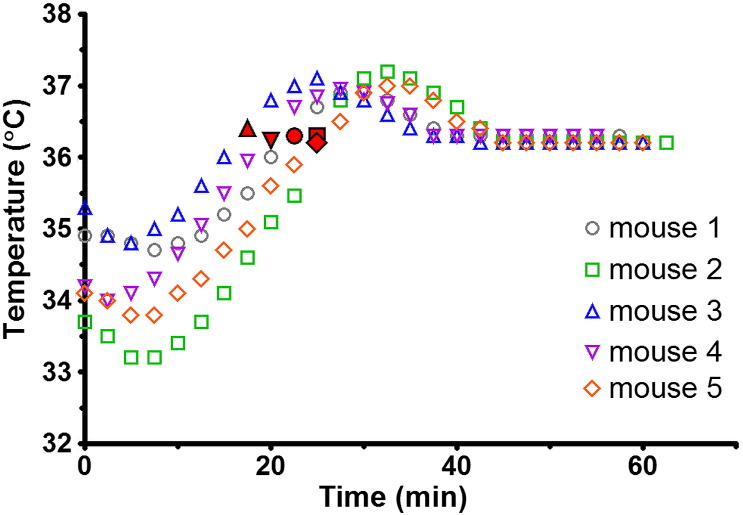
The results of the heat-loss recovery test, studying the recovery of the animal's heat loss upon anesthetic induction using a DCE-MRI type scan as an additional heat input, are presented in Fig. 4. The unpredictable temperature loss of up to 3 °C during induction of anesthesia, animal preparation and animal positioning was recovered within 15–25 minutes. Again, when the DCE-MRI type scan was stopped, the core temperature reached 36.3 °C for a target set to 37 °C.
Fig. 4.
Heat-loss recovery test. Temperature maintenance of 5 nude mice following induction of anesthesia and animal positioning using a DCE-MRI type scan as an additional heat source to the twisted pair heater wire. The red symbols indicate the time point at which the DCE-MRI type scan was stopped.
4. Discussion
Hypothermia in small rodents during anesthesia is an important complication and its negative consequences impact severely upon animal physiology, experimental reproducibility and, by implication, upon data quality. Consequently, maintenance of normothermia during anesthesia is critically important, but the range of devices suitable for achieving this effectively in the MRI scanner is limited, and data on their use and effectiveness are sparse [8]. Circulating warm air and liquids are commonly used in MRI [10,12]. However, they require space which may not be available when optimal RF coil loading and signal-to-noise ratios for high sensitivity MRI are required.
Commercially and home-built circulating fluid heating systems for MRI have been described elsewhere [12], and we add to the range of options for maintaining animals' temperature in the magnet. Our system is very easy and cheap to manufacture requiring just the production of a twisted copper wire and the insertion, in series, of a power resistor into the power line. These modifications are attached to a standard commercial homeothermic controller unit as used as standard in in vivo biology laboratories.
The proposed electrical resistive heater system was shown to be MR-compatible, and the low heat capacity of the cradle heater ensures a low thermal hysteresis. Use of the twisted wire arrangement led to self-cancellation of unwanted stray magnetic fields arising from the heater, and maintained B0 homogeneity. Furthermore, no interactions with the RF or gradient coils were observed. This set-up has allowed the use of mice weighing in excess of 30 g to be imaged within a 25 mm diameter coil and maximized the filling factor and SNR for these mice.
The quality of homeothermic maintenance using the twisted wire was evaluated in nude mice. The 2 W of heating was not only sufficient to recover the animal's heat loss upon induction of anesthesia but was also able to maintain the animal's body temperature to any single target temperature in the range of 34–38 °C. The homeothermic system sufficiently controlled the mouse's core temperature and overt hyperthermia could even be avoided when using a high duty cycle DCE-MRI type scan. The increase in core temperature resulting from the additional heat load of high duty cycle scanning was limited to 0.6–1.0 °C. Small animal body temperature dropped by several degrees C during anesthetic induction and animal positioning in the imaging cradle which is concurrent with other reports [11]. Although this temperature loss was recoverable, it implies that that any temperature-dependent scan modes, including blood flow weighted imaging, should be delayed until the animal has reached its target temperature. However, this waiting time can be reduced significantly if scanning is used as an additional heat input to increase throughput in routine experimentation.
5. Conclusions
The tightly wound twisted pair resistor requires no additional space, provides adequate heating capacity without causing any observable distortion of the magnetic field or the images, and can be implemented very easily.
Acknowledgments
This work was supported by Cancer Research UK and the Medical Research Council (C5255/A12678 and C2522/A10339). The sponsors were not involved in any part of the study design, in data collection, analysis and interpretation, in writing the report or in deciding to submit this work for publication.
Contributor Information
Veerle Kersemans, Email: Veerle.Kersemans@oncology.ox.ac.uk.
Stuart Gilchrist, Email: Stuart.Gilchrist@oncology.ox.ac.uk.
Philip D. Allen, Email: Danny.Allen@oncology.ox.ac.uk.
John S. Beech, Email: John.Beech@oncology.ox.ac.uk.
Paul Kinchesh, Email: Paul.Kinchesh@oncology.ox.ac.uk.
Borivoj Vojnovic, Email: Boris.Vojnovic@oncology.ox.ac.uk.
Sean C. Smart, Email: Sean.Smart@oncology.ox.ac.uk.
References
- 1.Sheffield C.W., Sessler D.I., Hunt T.K. Mild hypothermia during isoflurane anesthesia decreases resistance to E. coli dermal infection in guinea pigs. Acta Anaesthesiol Scand. 1994;38:201–205. doi: 10.1111/j.1399-6576.1994.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 2.Torossian A., Ruehlmann S., Middeke M., Sessler D.I., Lorenz W., Wulf H.F. Mild preseptic hypothermia is detrimental in rats. Crit Care Med. 2004;32:1899–1903. doi: 10.1097/01.ccm.0000139608.34486.fd. [DOI] [PubMed] [Google Scholar]
- 3.Flecknell P.A. Academic Press; San Diego (CA): 1996. Laboratory animal anaesthesia. [Google Scholar]
- 4.Otto K., von Thaden A. Chapter 5.4: anaesthesia, analgesia and euthanasia. In: Hedrich H.J., editor. The Laboraty Mouse. Academic Press; London: 2012. pp. 739–759. [Google Scholar]
- 5.Gordon C.J. Cambridge University Press; Cambridge: 1993. Temperature regulation in laboratory rodents. [Google Scholar]
- 6.Caro A.C., Hankenson F.C., Marx J.O. Comparison of thermoregulatory devices used during anesthesia of C57BL/6 mice and correlations between body temperature and physiologic parameters. J Am Assoc Lab Anim Sci. 2013;52:577–583. [PMC free article] [PubMed] [Google Scholar]
- 7.Hampshire V., Davis J., McNickle C. Red-carpet rodent care: making the most of dollars and sense in the animal facility. Lab Anim (NY) 2000;29:40–46. doi: 10.1038/5000057. [DOI] [PubMed] [Google Scholar]
- 8.Taylor D.K. Study of two devices used to maintain normothermia in rats and mice during general anesthesia. J Am Assoc Lab Anim Sci. 2007;46:37–41. [PubMed] [Google Scholar]
- 9.Campos F., Perez-Mato M., Agulla J., Blanco M., Barral D., Almeida A. Glutamate excitoxicity is the key molecular mechanism which is influenced by body temperature during the acute phase of brain stroke. PLoS One. 2012;7:e44191. doi: 10.1371/journal.pone.0044191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McConville P. Small animal preparation and handling in MRI. Methods Mol Biol. 2011;771:89–113. doi: 10.1007/978-1-61779-219-9_5. [DOI] [PubMed] [Google Scholar]
- 11.Kersemans V., Allen P.D., Beech J., Gilchrist S., Kinchesh P., Smart S. ISMRM; Milan, Italy: 2014. Application of prospective cardio-respiratory gating for simultaneous quantitative DCE-MRI of multiple mammary tumours in the mouse. [Google Scholar]
- 12.Murase K., Assanai P., Takata H., Saito S., Nishiura M. A simple and inexpensive system for controlling body temperature in small animal experiments using MRI and the effect of body temperature on the hepatic kinetics of Gd-EOB-DTPA. Magn Reson Imaging. 2013;31:1744–1751. doi: 10.1016/j.mri.2013.08.005. [DOI] [PubMed] [Google Scholar]