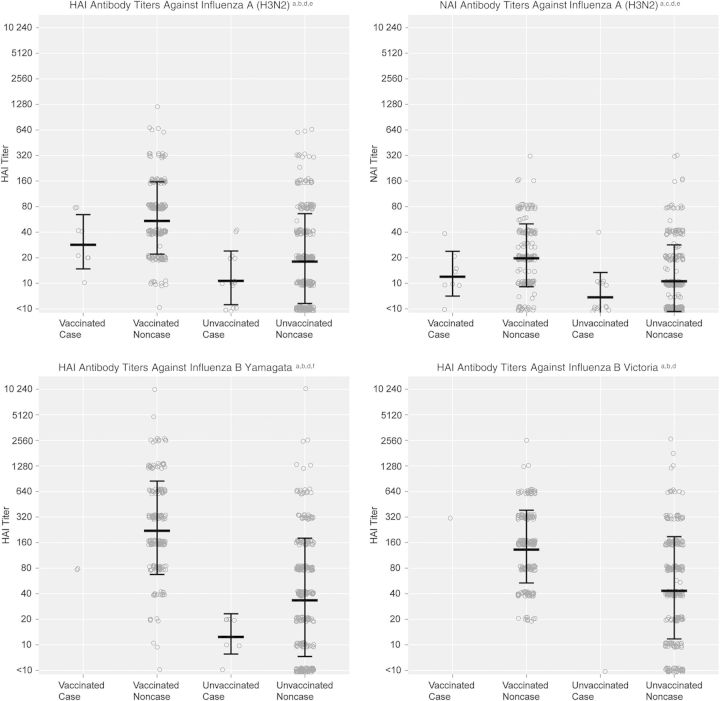
Figure 2.
Preseason susceptibility, based on antibody titers measured by hemagglutination inhibition (HAI) and neuraminidase inhibition (NAI) assays, in subjects, by influenza vaccination and infection status. aAntibody titers measured by HAI and NAI assays in sera collected from a subset of subjects aged ≥13 years at preseason visits (or at enrollment, for subjects without preseason specimens and no evidence of influenza vaccine receipt) were used to estimate preseason susceptibility to influenza; bSera were tested with the HAI assay, using as antigens the virus strains present in the 2012–2013 North American influenza vaccine (A[H1N1]: A/California/07/2009; A[H3N2]: A/Texas/50/2012 [A/Victoria/361/2011-like]; B/Yamagata: B/Texas/20/2011 [B/Wisconsin/1/2010-like]) plus the alternative influenza B lineage not contained in the vaccine (B Victoria: B/Brisbane/60/2008); cSera were tested with the NAI assay, using the influenza A(H3N2) strain present in the 2012–2013 North American influenza vaccine (A/Texas/50/2012 [A/Victoria/361/2011-like]) as the antigen; dEach circle indicates the titer of an individual observation. Linked lines indicate the geometric mean titer ± the geometric standard deviation; eInfluenza A(H3N2) cases had significantly lower geometric mean HAI and NAI titers than noncases overall (P = .02 and P = .02, respectively); this pattern was consistent for both vaccinated subjects (P = .06 and P = .09, respectively) and unvaccinated subjects (P = .17 and P = .07, respectively); fInfluenza B/Yamagata cases had significantly lower geometric mean HAI titers than noncases overall (P = .01); this pattern was consistent for both vaccinated subjects (P = .13) and unvaccinated subjects (P = .11).