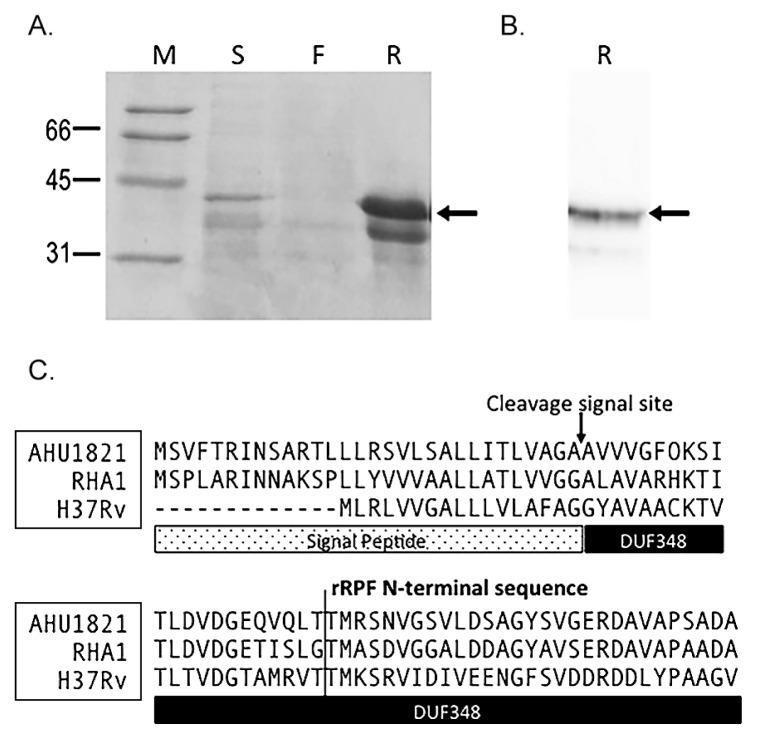
Fig. 1.
Expression and purification of the histidine-tagged recombinant Rpf (rRpf) protein from Rhodococcus erythropolis M1218 confirmed by SDS-PAGE (A) and Western blot hybridization analysis using an anti-His antibody (B). Arrows in panel A, B show the 39 kDa rRpf variant protein. M; molecular weight standard, R; histidine-tagged recombinant Rpf protein fraction, S; culture supernatant, F; flow through the fraction in the purification step. (C) Alignment of the N-terminal sequence of Rpf from Tomitella biformata AHU1821, Rhodococcus jostii RHA1, and Mycobacterium tuberculosis H37Rv with the signal peptide and DUF348 (Rpf conserved domain of unknown funtion) regions highlighted below the sequence. The arrow marks the theoretical signal cleavage site determined using SignalP 4.0 (21) and the line marks the terminal sequence of the two variants of the recombinant Rpf protein.