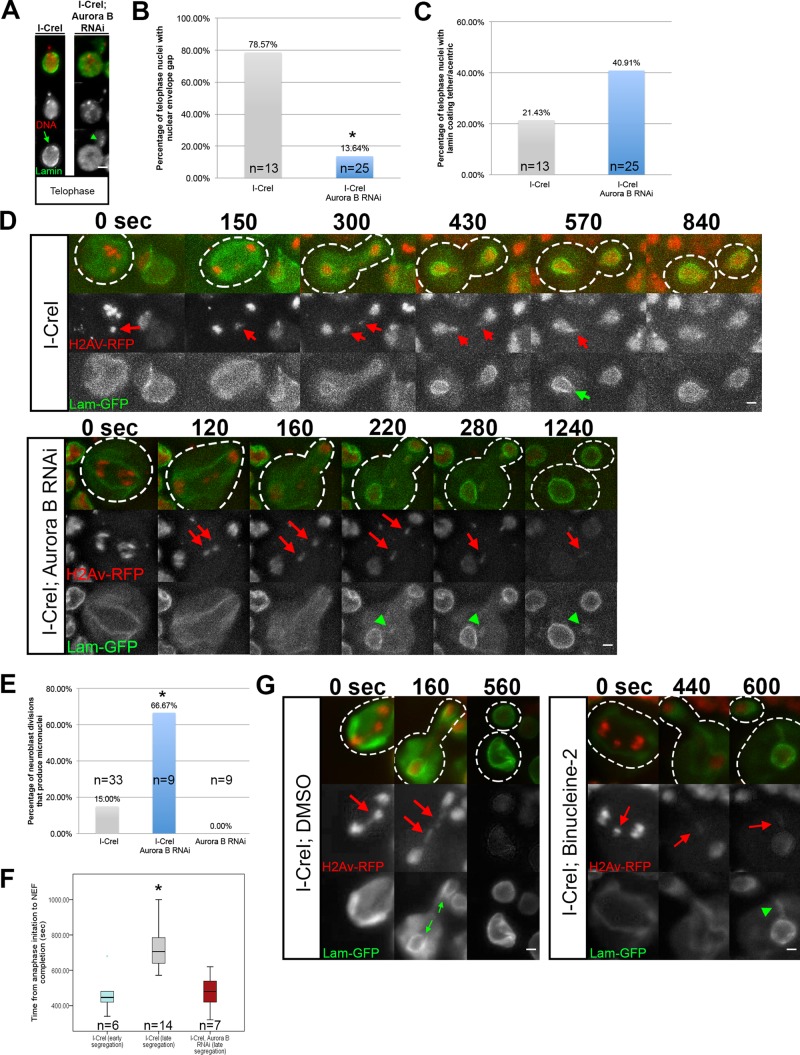
FIGURE 6:
Local inhibition of nuclear envelope assembly is mediated by Aurora B kinase. (A) Fixed images of telophase nuclei from I-CreI–expressing control and I-CreI with Aurora B depleted–neuroblasts bearing I-CreI–induced acentrics stained with anti-lamin (green) and DAPI (red). Acentrics induced in I-CreI–expressing controls show little to no association with lamin B and form a nuclear envelope gap (green arrow). In contrast, acentrics in neuroblasts with reduced Aurora B result in both the absence of a nuclear envelope gap and clear associations with lamins on acentrics (green arrowheads). (B) I-CreI–induced acentrics in control neuroblasts resulted in nuclear envelope gaps in 79% (N = 13) of telophase nuclei. In contrast, acentrics in neuroblasts depleted of Aurora B resulted in a significant reduction (14%; p < 0.05; N = 25) in the formation of nuclear envelope gaps in telophase nuclei. (C) Acentrics in I-CreI–expressing control neuroblasts were coated with lamin B in 21% (N = 13) of telophase nuclei. In contrast, telophase nuclei of neuroblasts depleted of Aurora B resulted in an increase (41%; N = 25) of acentrics coated with lamins (not significant). (D) Time-lapse images of dividing neuroblasts from I-CreI–expressing controls (top) and I-CreI with Aurora B RNAi (bottom; Supplemental Video S5) expressing lamin B-GFP (green) and H2Av-RFP (red). I-CreI–induced acentrics are present in both. In I-CreI–expressing controls, acentrics (red arrows) enter telophase nuclei through nuclear envelope gaps (green arrow). In contrast, neuroblasts bearing acentrics with reduced Aurora B show no gaps. Consequently, acentrics (red arrows) in Aurora B–depleted neuroblasts remain outside of the nucleus and form micronuclei coated with lamin B (green arrowheads). (E) Bar graphs of micronuclei frequencies in I-CreI–expressing control, I-CreI with Aurora B RNAi, and Aurora B RNAi without I-CreI control neuroblasts. (F) Box plots showing the time elapsed from the onset of anaphase to the completion of NEF in neuroblasts expressing I-CreI and I-CreI with Aurora B RNAi. Early-segregating I-CreI–induced acentrics do not form gaps, whereas late-segregating I-CreI–induced acentrics do form gaps. Late-segregating acentrics in neuroblasts with reduced Aurora B expression fail to form gaps and complete NEF in a similar time frame to early-segregating wild-type acentrics. These results indicate that Aurora B mediates the local inhibition of NEF in neuroblasts. Asterisks indicate statistical significance. (G) Time-lapse images of dividing neuroblasts from I-CreI–expressing, dimethyl sulfoxide (DMSO)–treated controls (left) and I-CreI–expressing Binucleine-2–treated neuroblasts (right) expressing lamin B-GFP (green) and H2Av-RFP (red). In DMSO-treated controls, acentrics (red arrows) enter telophase nuclei through nuclear envelope gaps (green arrows). In contrast, Binucleine-2–treated neuroblasts show no gaps. Consequently, acentrics (red arrows) in Aurora B–depleted neuroblasts remain outside of the nucleus and form micronuclei coated with lamin B (green arrowhead).