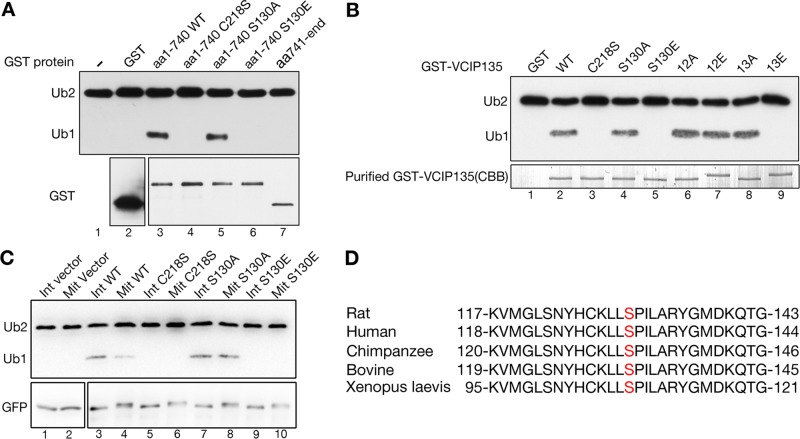
FIGURE 2:
Phosphorylation of VCIP135 at S130 inhibits its deubiquitinase activity. (A) Purified GST or GST-VCIP135 recombinant proteins were incubated with K48-linked di-ubiquitin; this was followed by Western blotting for ubiquitin and GST. Note that the WT N-terminal fragment (aa 1–740) of VCIP135 and its S130A mutant cleaved K48 di-ubiquitin into monoubiquitin, but its C218S enzyme-dead mutant and the S130E phosphomimetic mutant did not. (B) GST or GST-tagged full-length VCIP135 or its mutants were incubated with K48-linked di-ubiquitin followed by Western blotting for ubiquitin. The Coomassie brilliant blue–stained gel showed the amount of GST-VCIP135 proteins used in this experiment. Note that all S130E-containing mutants (S130E and 13E) had no activity. (C) HeLa cells transfected with a GFP vector or indicated VCIP135-GFP constructs were either asynchronous (Int) or synchronized with nocodazole into mitosis (Mit). Cells were lysed, and VCIP135 was immunoprecipitated using a GFP antibody followed by a deubiquitinase assay with K48-linked di-ubiquitin. Note that the enzyme-dead C218S mutant of VCIP135 and the phosphomimetic mutant S130E did not cleave di-ubiquitin in either interphase and mitosis, while the phosphodeficient S130A mutant remained active even in mitosis. (D) Alignment of VCIP135 protein sequences from different species to show the conservation of S130 (highlighted in red).