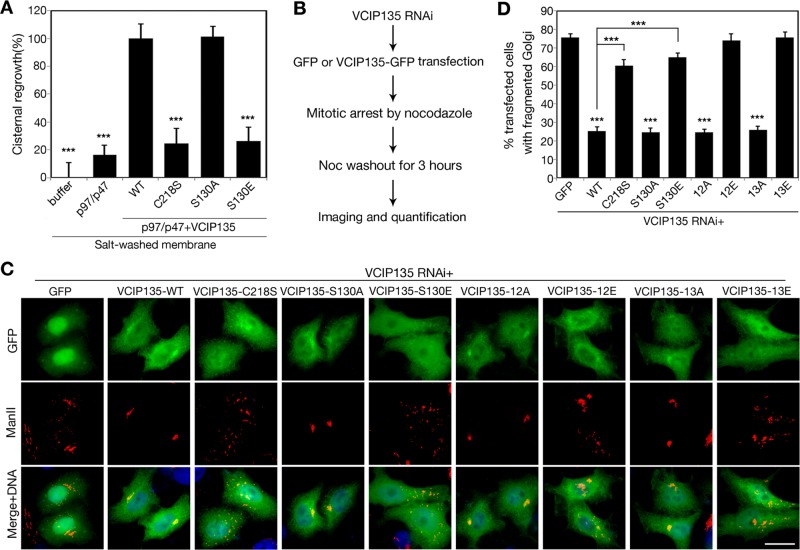
FIGURE 7:
Phosphorylation of VCIP135 on S130 attenuates p97/p47-mediated Golgi membrane fusion. (A) RLG membranes were fragmented by incubation with mitotic cytosol. The MGFs were washed with a high-salt buffer to remove endogenous VCIP135 and then incubated with p97/p47 alone or in the presence of SBP-tagged WT VCIP135 or indicated mutants. Samples were processed for EM and quantified for cisternal membrane regrowth (mean ± SEM). 0% represents MGF incubated with buffer alone; and 100% represents MGFs incubated with p97/p47 and WT VCIP135. The statistical significance of the results was assessed by Student's t test in comparison with the result with p97/p47 and WT VCIP135. ***, p < 0.001. Note that WT VCIP135 and the S130A mutant but not the phosphomimetic S130E mutant restored Golgi fusion activity. (B) Schematic paradigm of the VCIP135 depletion and rescue assay. HeLa cells were first depleted of endogenous VCIP135 by RNAi, then transfected with indicated GFP or GFP-tagged RNAi-resistant VCIP135 constructs, arrested to mitosis by nocodazole treatment, and incubated with fresh growth medium for 3 h. Cells were fixed and stained for ManII and analyzed for Golgi morphology. (C) Representative fluorescence images of postmitotic cells expressing indicated constructs, as described in B. Note that the WT and S130A, 12A, and 13A mutants of VCIP135 supported postmitotic Golgi reassembly, while the inactive mutants C218S and S130E failed. Scale bar: 20 μm. (D) Quantitation of C from three sets of independent experiments for the percentage of GFP-positive cells with fragmented Golgi. Results are expressed as the mean ± SEM. p Value shows the significance when cells expressing VCIP135-GFP were compared with cells expressing GFP alone, unless indicated.