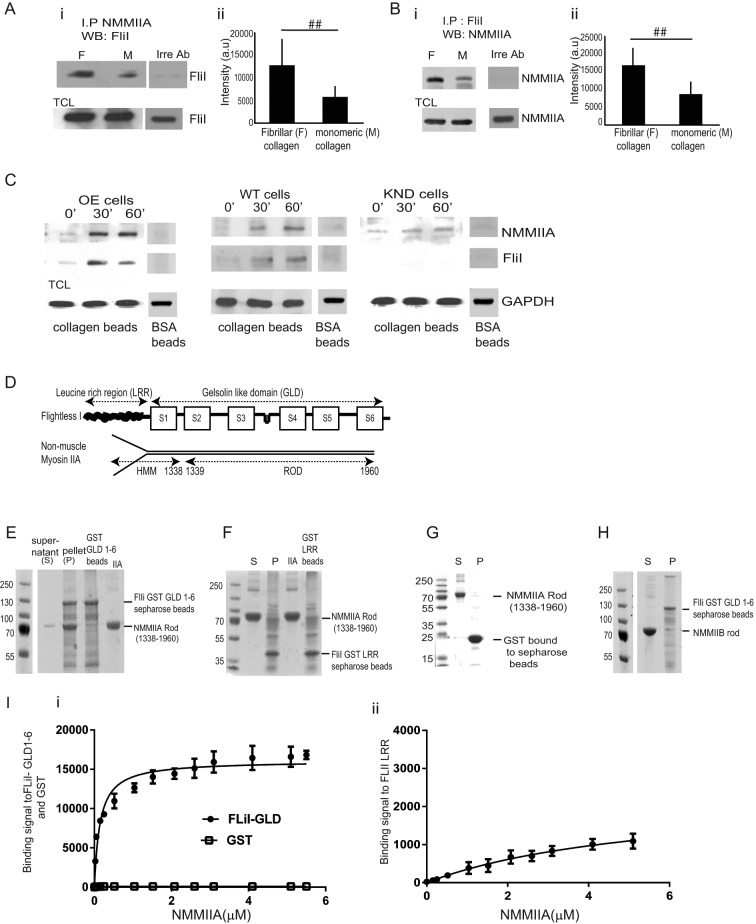
FIGURE 5:
FliI interacts with NMMIIA. (A, B) (i, ii) NMMIIA, FliI, or an irrelevant antibody (nebulin) was used to immunoprecipitate proteins on cells plated on fibrillar or monomeric collagen and then immunoblotted for FliI and NMMIIA, respectively. Histograms show quantification of mean blot density of immunoblots. There was twofold reduction of FliI and NMMIIA interaction in cells plated on monomeric collagen. These experiments were repeated four times. Blot density reported as mean ± SD and analyzed by ANOVA. ##p < 0.01, comparison of monomeric collagen to fibrillar collagen in NMMIIA and FliI immunoprecipitates. (C) FliI OE, WT, and KND cells incubated with collagen-coated beads for 0, 30, and 60 min. Beads were isolated, and bead-associated proteins (FLiI and NMMIIA) were examined by immunoblotting. (D) Structural components of FliI and NMMIIA. (E) Pull-down assays show pelleting of NMMIIA rods (1338–1960 aa) with GST-FliI GLD 1–6. (F) Pull-down assays show that NMMIIA rods (1338–1960 aa) do not associate with GST-FliI LRR Sepharose beads. (G) Control pull-down assay shows that GST does not associate with myosin rods. (H) Pull-down assays show limited binding of NMMIIB with FliI GLD 1–6. (I) (i, ii) Analysis of pull-down experiments show quantification of binding of FliI GLD 1–6 to NMMIIA (kD = 0.146 μM) compared with FliI LRR region (kD = 5.074 μM).