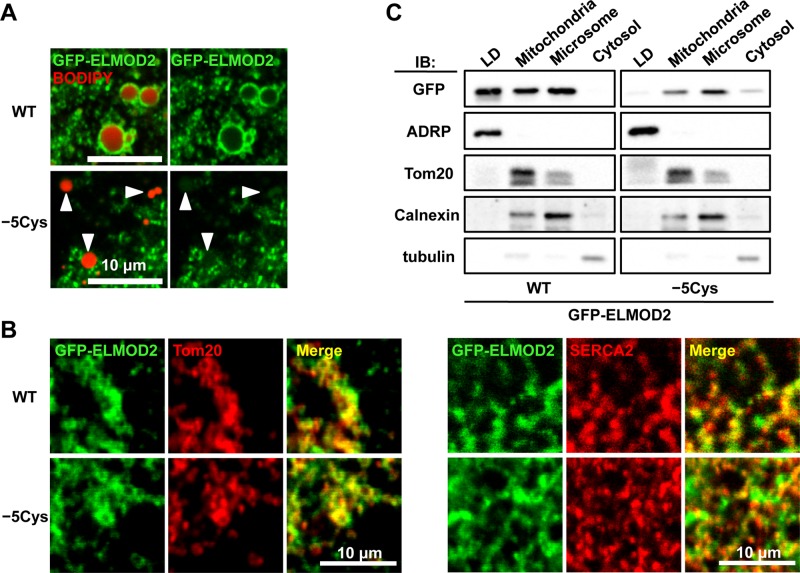
FIGURE 4:
Palmitoylation and subcellular distribution of ELMOD2. (A) Replacement of five cysteine residues with alanine significantly decreased the accumulation of ELMOD2 in LDs. Huh7 cells expressing either GFP-ELMOD2 (WT) or the mutant, GFP-ELMOD2 (−5Cys) (green) were incubated with BODIPY558/568-C12 to label LDs (red; arrowheads) and treated with 0.1% saponin for 5 min before fixation to extract cytosolic proteins. (B) GFP-ELMOD2 (−5Cys) as well as GFP-ELMOD2 (WT) (green) showed colocalization with SERCA2 and Tom20 (red), indicating distribution in the ER and mitochondria, respectively. (C) Huh7 cells expressing either GFP-ELMOD2 (WT) or GFP-ELMOD2 (−5Cys) were fractionated by ultracentrifugation and analyzed by Western blotting. GFP-ELMOD2 (−5Cys) lacking palmitoylation was scarcely found in the LD fraction but was observed in the mitochondria and microsomal fractions. Consistent with the decrease in the LD fraction, the proportion of GFP-ELMOD2 (−5Cys) in the cytosol was increased in comparison with that of GFP-ELMOD2 (WT). The expression level of GFP-ELMOD2 (−5Cys) was lower than that of GFP-ELMOD2 (WT), as seen previously for other palmitoylated proteins (Tanimura et al., 2006). ADRP, Tom20, calnexin, and tubulin were used as markers of LDs, mitochondria, microsome, and cytosol, respectively.