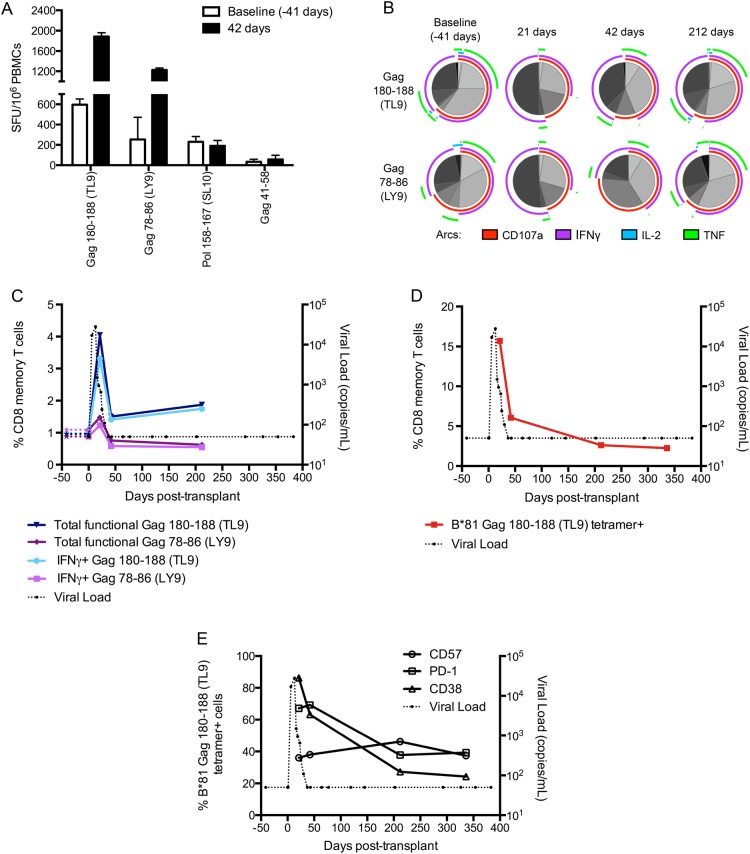
Figure 3.
Human immunodeficiency virus type 1 (HIV-1)–specific CD8+ T-cell responses are highly functional, expanding strongly with control of HIV-1 reactivation. T-cell responses were initially mapped by interferon-gamma (IFN-γ) enzyme-linked immunospot at +336 days and were refined to optimal epitopes based on the patient's human leukocyte antigen type. A, IFN-γ responses to mapped peptides were assayed in the baseline sample and peripheral blood mononuclear cells (PBMCs) at +42 days post-transplantation. Results represent mean ± standard deviation spot forming units/106 (SFU) cells of triplicate measurements. B, Functionality of TL9 and LY9 CD8 memory T-cell responses before (−41 days, viral load [VL] <50), during (+21 days, VL measured at +19 and +23 days was 940 and 650 copies/mL, respectively), and after (+42 and +212 days, VL < 50) virus rebound. Colored arcs indicate cytokines. Shaded sectors show the proportion of each cytokine combination. C, Gag 180–188 (TL9) and Gag 78–86 (LY9) T-cell responses measured by intracellular cytokine flow cytometry prior to and during VL rebound. Percentage of CD8+ memory T cells expressing 1 or more of IFN-γ, tumor necrosis factor (TNF)-alpha, interleukin-2 (IL-2), and CD107a, as well as IFN-γ only, shown. D, Kinetics of B*81:01 Gag180–188 tetramer+ cells and VL during the study period. E, Changes in expression of activation markers CD57, PD-1, and CD38 by tetramer+ CD8 memory T cells during VL decline. Note: PBMCs prior to chemotherapy were unavailable; however, a sample from the leukapheresis at day −41 that was transplanted at day 0 was considered to be a baseline and representative of HIV-1–specific T-cell responses prior to chemotherapy.