Abstract
This biofilm study was conducted to assess the in vitro activity of tetrasodium EDTA on catheters that had been routinely removed from hemodialysis patients at Leeds Teaching Hospitals Trust due to maturation of fistula. Catheters were screened by culture of through-catheter flush, and isolates were identified by standard methodologies; 20 isolates were found to be biofilm positive. Initial biofilm cell count levels averaged above 105 CFU/1-cm catheter section. Bacteria identified in the biofilms were gram-negative (1 isolate), gram-positive (11 isolates), or mixed species (8 isolates). After a 24-h lock, 40 mg of tetrasodium EDTA per ml was effective at eradicating the total biofilm viable count in almost all cases. The efficacy of tetrasodium EDTA as a catheter lock potentially shows that this agent could substantially reduce catheter-related infections and be used to treat patients with limited access.
Catheters have become essential in the management of critical-care patients, yet the inside of a catheter is a major source of infection due to biofilms (1, 3). It has been found that at least 70% of all nosocomial bloodstream infections occur in patients with central venous catheters (7). Traditionally, catheters have been locked with normal saline or heparin solutions, sometimes in combination, to provide anticoagulant activity. Normal saline is generally used to lock short-term peripheral intravenous catheters, but saline has no anticoagulant or antimicrobial activity. Heparin solutions are generally used to lock vascular catheters. Heparin has anticoagulant activity, but it does not function as an antimicrobial and therefore does not provide protection from catheter-related infections. Recently, antibiotic lock solutions, with or without an anticoagulant (such as vancomycin-heparin), have been used for the prevention and management of catheter-related bacteremia (2, 8, 15). However, there are concerns, particularly with the use of a vancomycin flush solution, because of the potential for the development of resistant organisms (5, 9).
EDTA is a calcium and iron chelator with anticoagulant activity. However, as a disodium salt it has been documented to have limited antistaphylococcal and anti-Candida activities (6, 12, 13). In contrast to this was a study by Raad et al., who demonstrated that minocycline and EDTA have highly synergistic activities in the decontamination of catheter surfaces when they are combined in a solution (10). It was found in that study that minocycline-EDTA was active against staphylococci, gram-negative bacilli, and Candida organisms that colonize polymers. This suggests a possible role of EDTA in catheter lock procedures.
We undertook the present study by using in vivo renal lines in an in vitro technique to test the efficacy of tetrasodium EDTA on catheters removed from patients in order to determine its potential as a catheter lock solution to treat preformed biofilms and, ultimately, catheter-related infections.
MATERIALS AND METHODS
Experimental design.
The study was conducted at the Leeds Teaching Hospitals Trust, Leeds, United Kingdom. Renal dual-lumen circle C advantage hemodialysis catheters (Horizon Medical Products, Manchester, United Kingdom), collected after routine removal from patients, were evaluated for biofilm prevalence. Twenty catheters were found to be positive for evidence of biofilms, and these were utilized for evaluating the effectiveness of tetrasodium EDTA on biofilms.
Screening protocol.
The inner lumens of all catheters, aseptically removed from the patient, were slowly flushed with 1 ml of sterile phosphate-buffed saline to remove the high numbers of planktonic bacteria and confirm the presence of biofilm. Duplicate 10-μl aliquots of the flushed lumen samples were spread plated onto fresh blood agar plates (Oxoid, Basingstoke, United Kingdom) and incubated at 37°C for 48 h. The samples were vortexed for 1 min prior to plating out. After flushing (screening), all catheters were sterilized on the outer surface with alcohol wipes and then were stored at 4°C, in sterile specimen bags, until the results of the screen were available. All external surfaces of catheters were found to be negative for the presence of contaminating bacteria after cleaning and sterilization. Sections of the inner lumens of catheters were viewed under scanning electron microscopy (SEM) to confirm biofilm presence. Noncolonized catheter lines were discarded. All isolated cultures were identified by using Gram staining, microscopy, catalase, coagulase, methicillin strips, and API 20E and API 20NE kits (BioMerieux, Marcy l'Etoile, France).
Lock protocol.
Prior to a lock treatment test with 40 mg of tetrasodium EDTA (Sigma-Aldrich, Dorset, United Kingdom) per ml, the catheters which were positive for sessile bacteria were locked with 5 ml of nutrient broth in each lumen, administered with a 5-ml syringe, and incubated overnight at 37°C. Following incubation, each catheter lumen was slowly flushed with 5 ml of phosphate-buffered saline (as described above), and two 1-cm pieces were cut from the distal end with a sterile scalpel blade. One piece, neutralized in 1 ml 1 of M calcium chloride (Sigma-Aldrich), was used for culture and bacterial identification, and the other catheter section was stored in 4% formal saline prior to SEM analysis. The catheters were then locked with 40 mg of tetrasodium EDTA lock fluid per ml for up to 24 h. After 3 h, two 1-cm sections were cut from each catheter, using a sterile scalpel blade, in a biological safety cabinet to avoid exogenous contamination. One catheter section was neutralized in 1 ml of 1 M calcium chloride for culturing, and the other catheter section was placed in 4% formal saline for SEM analysis. This same procedure was carried out at 6 and 24 h.
Biofilm removal.
Following treatment and the appropriate incubation periods, the catheter sections that were placed in 1 ml of 1 M calcium chloride were sonicated in a sonicating water bath (DP 200-00; Ultrawave, Cardiff, United Kingdom) for 15 min and plated out onto fresh blood agar for colony counts. This procedure was repeated for all lock times. Fifteen minutes of sonication was found to be the most effective time for maximum biofilm removal (data not shown).
After a 24-h lock, both lumens of the catheters were brushed with an endoluminal brush (model RC4060/5; FAS Medical International) to confirm the absence of viable organisms. Each brush was added to 1 ml of CaCl (1 M), sonicated in a water bath for 15 min, plated out onto fresh blood agar, and incubated overnight at 37°C. SEM was used to confirm biofilm presence on all catheters after brushing.
SEM.
Catheter sections were cut into small sections and fixed by placing them in 4% formal saline overnight at room temperature. The samples were then dehydrated in a graded series of ethanol solutions (20, 40, 60, and 80%) for 15 min each at room temperature, immersed in acetone for 4 h at room temperature, and dehydrated overnight by using a critical-point dryer. Finished specimens were mounted on aluminum stubs with carbon paint, sputter coated with 25-nm-diameter gold particles (Polaron E 5300), and examined with a CamScan series III SEM (Obducat Camscan Ltd., Cambridge, United Kingdom).
RESULTS AND DISCUSSION
Effectiveness of tetrasodium EDTA on monoculture biofilms.
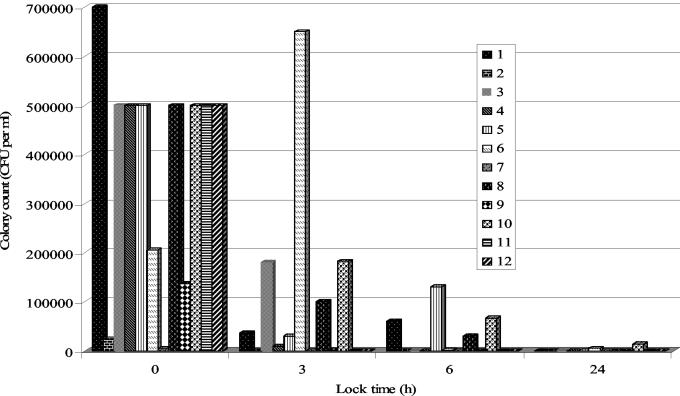
Biofilm, confirmed by viable cell counts and SEM, was clearly evident on all catheters removed from patients. The biofilms detected had viable cell counts ranging from log 4 to log 6 CFU per ml, as shown in Fig. 1. With these particular patients, the predominant species of bacteria isolated from the catheters included methicillin-resistant Staphylococcus aureus (MRSA), coagulase-negative Staphylococcus, Enterobacter, and Enterococcus spp., as shown in Table 1. After 3 h, the effectiveness of tetrasodium EDTA was found to significantly (P < 0.05) reduce the sessile viable population by over 30% in the majority of cases. After 6 h, the biofilm had been reduced by over 80% of it original viable cell count, and finally, after 24 h, tetrasodium EDTA, in the majority of cases, had effectively eradicated the biofilm.
FIG. 1.
In vivo renal lines in an in vitro technique to test the efficacy of tetrasodium EDTA (40 mg/ml) in removing monospecies bacterial biofilm. Colony counts over time for catheters 1 to 12 (Table 1) are shown.
TABLE 1.
Microbiology of biofilms removed from hemodialysis catheters
| Catheterno. | Microorganism(s) |
|---|---|
| 1 | MRSA |
| 2 | MRSA |
| 3 | Coagulase-negative Staphylococcus |
| 4 | Coagulase-negative Staphylococcus |
| 5 | S. aureus |
| 6 | Enterococcus sp. |
| 7 | MRSA |
| 8 | Coagulase-negative Staphylococcus |
| 9 | MRSA |
| 10 | Coagulase-negative Staphylococcus |
| 11 | Enterobacter cloacae |
| 12 | Coagulase-negative Staphylococcus |
| 13 | Pseudomonas aeruginosa, Enterococcus sp., E. cloacae, coagulase-negative Staphylococcus, MRSA |
| 14 | P. aeruginosa, Enterococcus sp., E. cloacae, coagulase-negative Staphylococcus, MRSA |
| 15 | Coagulase-negative Staphylococcus, coryneforms |
| 16 | Streptococcus sp., coagulase-negative Staphylococcus, gram-negative bacilli (oxidase positive) |
| 17 | Klebsiella pneumoniae, coagulase-negative Staphylococcus |
| 18 | Pseudomonas sp., Streptococcus sp. |
| 19 | Coagulase-negative Staphylococcus, group D streptococci |
| 20 | Mixed coagulase-negative Staphylococcus |
Effectiveness of tetrasodium EDTA on mixed-species biofilm.
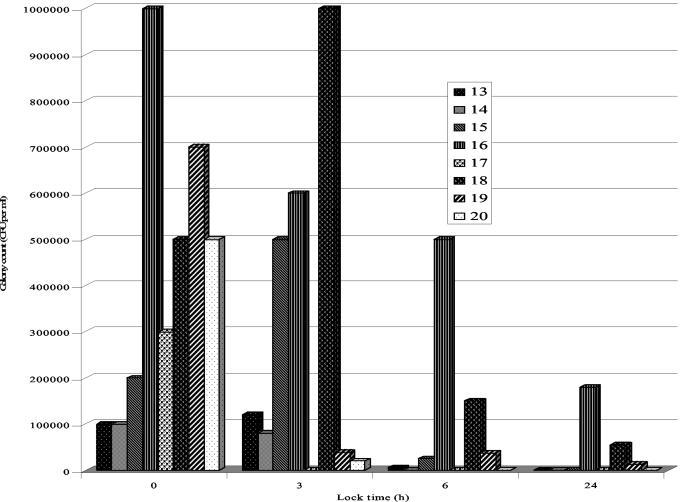
From Fig. 2 it is evident that the biofilm viable cell count ranged from log 5 to log 6 CFU per ml in catheters removed from patients. After a lock of 3 h, tetrasodium EDTA showed little effect on mixed-species biofilms, with a large increase in sessile counts in the biofilm containing Pseudomonas and Streptococcus (Fig. 2; Table 1). However, after 6 h, the effect of tetrasodium EDTA was clearly apparent, with a reduction of viable cell counts of over 50% of the original biofilm. After 6 h, the biofilm viable cell count decreased further, with no viable cell count in the majority of cases after a 24-h lock. Clearly, in all cases the action of tetrasodium EDTA had significantly (P < 0.05) reduced the biofilm viable cell count.
FIG. 2.
In vivo renal lines in an in vitro technique to test the efficacy of tetrasodium EDTA (40 mg/ml) in removing mixed-species bacterial biofilm. Colony counts over time for catheters 13 to 20 (Table 1) are shown.
Catheter-related infections due to biofilms remain a significant clinical problem in many institutions, and a lock solution, which can prevent or control the growth of biofilms in a catheter lumen, is imperative for the prevention of catheter-related bloodstream infections. Many studies have examined the prevention of endoluminal catheter-related infection based on catheter flushing, but these studies have shown conflicting results. For example Schwartz et al., using a solution of heparin and vancomycin to flush central venous catheters compared its efficacy with that of heparin (15). It was found in this study that daily flushing with heparin and vancomycin significantly decreased the frequency of catheter-related bacteremia attributed to luminal colonization.
Most catheters are colonized with organisms embedded in a biofilm, but infection appears to depend on whether the organisms on the catheter surface, particularly those in a planktonic free-floating phase, exceed a certain quantitative threshold. In the present study, which involved 20 catheters removed from hospitalized patients, a concentration of 40 mg of tetrasodium EDTA per ml was found to eradicate biofilm; this must significantly aid in the reduction of catheter-related infections. Tetrasodium EDTA was found to have a broad-spectrum inhibitory activity against MRSA and gram-negative bacilli.
Many studies have shown that a combination of minocycline and EDTA is uniquely useful in disrupting the biofilm and synergistically eradicating organisms from the biofilm environment (4, 11, 14). In a more recent study the combination of minocycline and EDTA flush solution was shown to be highly efficacious in preventing catheter-related colonization, bacteremia, septic phlebitis, and endocarditis in an animal model (10).
The multifocal mechanisms of action of tetrasodium EDTA in inhibiting biofilm formation primarily involve its metal-chelating activity. In this present study, which involved 20 catheters removed from hospitalized patients, a tetrasodium EDTA concentration of <40 mg/ml was found to eradicate biofilm, based on viable cell counts, in the majority of cases. Tetrasodium EDTA was found to have broad-spectrum activity against both gram-positive and gram-negative organisms in in vivo-generated biofilms.
Acknowledgments
We acknowledge the generous financial support of Aseptica Inc., Seattle, Wash.
REFERENCES
- 1.Anaissie, E., G. Samonis, D. Kontoyiannis, J. Costerton, U. Sabharwal, G. Bodey, and I. Raad. 1995. Role of catheter colonization and infrequent hematogenous seeding in catheter-related infections. Eur. J. Clin. Microbiol. Infect. Dis. 14:135-137. [DOI] [PubMed] [Google Scholar]
- 2.Carratala, J., J., Niubo, A. Fernandez-Sevilla, E. Juve, X. Castellsague, J. Berlanga, J. Linares, and F. Gudiol. 1999. Randomized, double-blind trial of an antibiotic-lock technique for prevention of gram-positive central venous catheter-related infection in neutropenic patients with cancer. Antimicrob. Agents Chemother. 43:2200-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christensen, G. D., L. Baldassari, and W. A. Simpson. 1994. Colonization of medical devices by coagulase-negative staphylococci, p. 45-78. In A. L. Bisno and F. A. Waldvogel (ed.), Infections associated with indwelling medical devices, 2nd ed. American Society for Microbiology, Washington, D.C.
- 4.Evans, R. C., and C. J. Holmes. 1987. Effect of vancomycin hydrochloride on Staphylococcus epidermidis biofilm associated with silicone elastomer. Antimicrob. Agents Chemother. 31:889-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garland, J. S., K. Henrickson, and D. G. Maki. 2002. The 2002 Hospital Infection Control Practices Advisory Committee, Centers for Disease Control and Prevention, guideline for prevention of intravascular device-related infection. Pediatrics 110:1009-1013. [DOI] [PubMed] [Google Scholar]
- 6.Gil, M. L., M. Casanova, and J. P Martinez. 1994. Changes in the cell wall glycoprotein composition of Candida albicans associated to the inhibition of germ tube formation by EDTA. Arch. Microbiol. 161:489-494. [DOI] [PubMed] [Google Scholar]
- 7.Haley, R., D. H. Culver, J. W. White W. M. Morgan, and T. G. Emori. 1985. The nationwide nosocomial infection rate: a new need for vital statistics. Am. J. Epidemiol. 121:159-167. [DOI] [PubMed] [Google Scholar]
- 8.Henrickson, K. J., R. A. Axtell, S. M. Hoover, S. M. Kuhn, J. K. Pritchett, S. C. Klein, and P. John. 2000. Prevention of central venous catheter-related infections and the thrombotic events in immunocompromised children by the use of vancomycin/ciprofloxacin/heparin flush solution: a randomized, multicenter, double-blind trial. J. Clin. Oncol. 18:1269-1278. [DOI] [PubMed] [Google Scholar]
- 9.Mermel, L. A. 2000. Prevention of intravascular catheter-related infections. Ann. Intern. Med. 132:391-402. [DOI] [PubMed] [Google Scholar]
- 10.Raad, I., R. Hachem, R. K. Tcholakian, and R. Sheretz. 2002. Efficacy of minocycline and EDTA lock solution in preventing catheter-related bacteremia, septic phlebitis, and endocarditis in rabbits. Antimicrob. Agents Chemother. 46:327-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raad, I., and R. Sherertz. November 1994. M-EDTA pharmaceutical preparations and uses thereof. U.S. patent 5,362,754.
- 12.Reardon, D. M., B. Warner, B., and E. A. Trowbridge. 1991. EDTA, the traditional anticoagulant of haematology: with increased automation is it time for review? Med. Lab. Sci. 48:72-75. [PubMed] [Google Scholar]
- 13.Root, J. L., O. R. McIntyre, N. J. Jacobs, and C. P. Daghlian. 1988. Inhibitory effect of disodium EDTA upon the growth of Staphylococcus epidermidis in vitro: relation to infection prophylaxis of Hickman catheters. Antimicrob. Agents Chemother. 32:1627-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rose, R. K. 2000. The role of calcium in oral streptococcal aggregation and the implications for biofilm formation and retention. Biochim. Biophys. Acta 1475:76-82. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz, C., K. J. Henrickson, K. Roghmann, and K. Powell. 1990. Prevention of bacteremia attributed to luminal colonization of tunneled central venous catheters with vancomycin-susceptible organisms. J. Clin. Oncol. 8:1591-1597. [DOI] [PubMed] [Google Scholar]