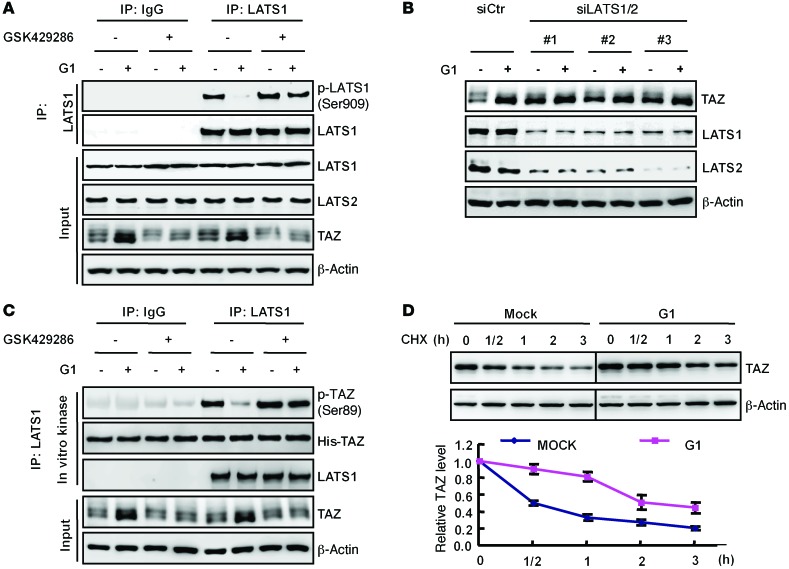
Figure 4. GPER activates TAZ via LATS inhibition.
(A) G1 inhibited the phosphorylation of LATS. Serum-starved ZR-75-30 cells were pretreated with 200 nM of the ROCK inhibitor GSK429286 for 4 hours, followed by treatment with 100 nM G1 for 2 hours. For detection of LATS1 phosphorylation, immunoprecipitated LATS1 was used for immunoblotting with p-LATS antibody. (B) LATS was required for G1-induced TAZ accumulation. LATS1/2 were knocked down by 3 independent siRNAs in ZR-75-30 cells. These cells were stimulated with 100 nM G1 as indicated. Protein levels of TAZ and the knockdown efficiency of LATS1/2 were assessed by immunoblotting. (C) LATS1 kinase activity was inhibited by G1 in a ROCK-dependent manner. ZR-75-30 cells were pretreated with the ROCK inhibitor GSK429286 or control, followed by a 2-hour treatment with 100 nM G1 as indicated. Immunoprecipitated LATS1 was subjected to an in vitro kinase assay using His-TAZ as a substrate. TAZ phosphorylation was detected by p-TAZ (Ser89) antibody. (D) G1 treatment stabilized TAZ protein. Serum-starved ZR-75-30 cells were pretreated with mock or 100 nM G1 for 2 hours and then treated with CHX (20 μg/ml) for the indicated durations. The amounts of TAZ were analyzed by immunoblotting and quantified by densitometry and normalized to β-actin. Data are represented as the mean ± SD; n = 3. Blots shown are representative of at least 3 independent experiments.