Abstract
Accurate staging of esophageal cancer is very important to achieving optimal treatment outcomes. The AJCC (American Joint Committee on Cancer) first published TNM esophageal cancer staging recommendations in the first edition of their staging manual in 1977. Thereafter, the staging of esophageal cancer was changed many times over the years. This article reviews the current status of staging of esophageal cancer.
Keywords: Esophageal neoplasms, Neoplasm staging, Survival, Histologic grade
INTRODUCTION
Tumor-node-metastasis (TNM) cancer staging was developed to describe the anatomic extent of tumors by Pierre Denoix of the Institut Gustave-Roussy between 1943 and 1952. It is based on the principle that the as size of an untreated primary tumor (T) increases, first regional lymph node metastases (N) and then distant metastases (M) become more frequent. Although introduced in 1953, it was not until 1968 that the first cancer staging manual was published by the International Union Against Cancer (UICC).
The American Joint Committee on Cancer (AJCC) first published TNM esophageal cancer staging recommendations in the first edition of their staging manual in 1977. In 1988, the UICC and AJCC esophageal cancer staging guidelines were unified. Initially, the staging of esophageal cancer advanced rapidly, but unfortunately then stagnated for decades. Prior to the novel revision of esophageal cancer described in this paper, the criteria for T, N, and M classifications had not changed since 1988, 1977, and 1997, respectively. The long-held concept of stage groupings of esophageal cancer has been a hindrance to the evolution of esophageal cancer staging, because this concept is incorrectly based on a simple, orderly arrangement of increasing anatomic T, then N, then M classifications. This assumption is not consistent with cancer biology or survival data. Worldwide collaboration [1] has provided data for a novel modern machine-learning analysis [2] that has produced data-driven staging for cancers of the esophagus and esophagogastric junction [3]. This new system is the basis for the seventh editions of the AJCC and UICC Cancer Staging Manuals [4,5]. It is more representative of and consistent with the survival rates of patients who undergo esophagectomy for esophageal cancer, and incorporates changes that address some difficulties in empirical stage grouping and previous areas of disharmony with stomach cancer staging [6]. In addition, TNM classifications have been revised where data, analysis, and consensus have demonstrated a need for change. For the first time, nonanatomic cancer characteristics, such as primary cancer site (location), histologic grade (grade), and histopathologic type (cell type) have been incorporated in esophageal cancer staging. This has been problematic for the UICC, which has resisted the addition of nonanatomic cancer characteristics and data-driven staging because of their belief that the principles of anatomic TNM staging must be preserved.
DATA
At the request of the AJCC, the Worldwide Esophageal Cancer Collaboration was initiated in 2006. Thirteen institutions from five countries and three continents (Asia, Europe, and North America) submitted deidentified data by July 2007. A database of 4,627 esophagectomy patients who had not undergone induction or adjuvant therapy was created [1].
ANALYSIS
Multiple previously proposed revisions of esophageal cancer staging have examined goodness of fit or p-values to test for a statistically significant effect of stage on survival. Instead, the staging introduced in the seventh edition used random forest (RF) analysis, a machine learning technique that focuses on predictiveness for future patients [2]. RF analysis makes no a priori assumptions about patient survival, is able to identify complex interactions among variables, and accounts for nonlinear effects. It may be viewed as a backward analysis that determines the anatomic classifications (TNM) and nonanatomic cancer characteristics that are associated with specific survival groups.
The RF analysis employed in this process first isolated cancer characteristics of interest from other factors that influence survival by generating risk-adjusted survival curves for each patient. Unlike previous approaches that began by placing cancer characteristics into proposed groups, RF analysis produced distinct groups with monotonically decreasing risk-adjusted survival rates without regard to cancer characteristics. Subsequently, anatomic and nonanatomic cancer characteristics important for the composition of stage groups were identified within the groups produced by RF analysis. Finally, the principle of assuring homogeneity within groups guided both the amalgamation and segmentation of cancer characteristics between adjacent groups to arrive at the final stage groupings [3–5].
SEVENTH EDITION TNM CLASSIFICATIONS: CHANGES AND ADDITIONS
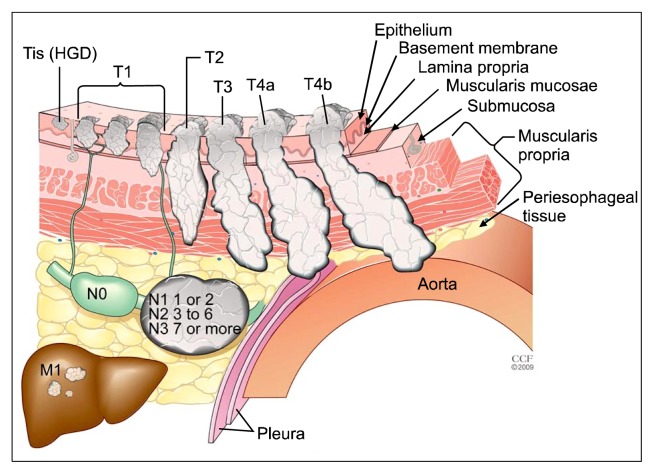
The primary tumor (T) classification has been changed for Tis and T4 cancers (Fig. 1, Table 1). Tis is now defined as high-grade dysplasia and includes all instances of noninvasive neoplastic epithelium that were previously called carcinoma in situ. The T4 category that includes tumors that invade local structures has been subclassified into T4a and T4b tumors; T4a tumors are resectable cancers invading adjacent structures such as the pleura, pericardium, or diaphragm, while T4b tumors are unresectable cancers invading other adjacent structures, such as the aorta, vertebral body, or trachea. Otherwise, the T classifications are unchanged (Fig. 1, Table 1).
Fig. 1.
Seventh edition TNM classifications. T is classified as follows: Tis, high-grade dysplasia (HGD); T1, cancer invades lamina propria, muscularis mucosae, or submucosa; T2, cancer invades muscularis propria; T3, cancer invades adventitia; T4a, resectable cancer invading adjacent structures such as pleura, pericardium, or diaphragm; and T4b, unresectable cancer invading other adjacent structures, such as the aorta, vertebral body, or trachea. The N classifications are as follows: N0, no regional lymph node metastasis; N1, regional lymph node metastases involving one to two nodes; N2, regional lymph node metastases involving three to six nodes; and N3, regional lymph node metastases involving seven or more nodes. M is classified as follows: M0, no distant metastasis; and M1, distant metastasis.
Table 1.
2010 seventh edition American Joint Committee on Cancer/International Union Against Cancer tumor-node-metastasis classifications
| Classification | Contents |
|---|---|
| Primary tumor (T) | TX: primary tumor cannot be assessed |
| T0: no evidence of primary tumor | |
| Tis: high-grade dysplasiaa) | |
| T1: tumor invades lamina propria, muscularis mucosae, or submucosa | |
| T1a: tumor invades lamina propria or muscularis mucosae | |
| T1b: tumor invades submucosa | |
| T2: tumor invades muscularis propria | |
| T3: tumor invades adventitia | |
| T4: tumor invades adjacent structures | |
| T4a: resectable tumor invading pleura, pericardium, or diaphragm | |
| T4b: unresectable tumor invading other adjacent structures, such as aorta, vertebral body, trachea, etc. | |
| Regional lymph nodes (N)b) | NX: regional lymph nodes cannot be assessed |
| N0: no regional lymph node metastasis | |
| N1: regional lymph node metastases involving 1 to 2 nodes | |
| N2: regional lymph node metastases involving 3 to 6 nodes | |
| N3: regional lymph node metastases involving 7 or more nodes | |
| Distant metastasis (M) | M0: no distant metastasis |
| M1: distant metastasis | |
| Histopathologic type | Squamous cell carcinoma |
| Adenocarcinoma | |
| Histologic grade (G) | GX: grade cannot be assessed—stage grouping as G1 |
| G1: well differentiated | |
| G2: moderately differentiated | |
| G3: poorly differentiated | |
| G4: undifferentiated—stage grouping as G3 squamous | |
| Locationc) | Upper or middle—cancers above lower border of inferior pulmonary vein |
| Lower—below inferior pulmonary vein |
Includes all non-invasive neoplastic epithelium that was previously called carcinoma in situ. Cancers stated to be non-invasive or in situ are classified as Tis.
Number must be recorded for total number of regional nodes sampled and total number of reported nodes with metastases.
Location (primary cancer site) is defined by position of upper (proximal) edge of tumor in esophagus.
A regional lymph node has been redefined to include any paraesophageal lymph node that extends from the cervical nodes to celiac nodes (Table 1). Analyses of the data support convenient coarse groupings of the number of cancer-positive nodes [2–4]. The regional lymph node (N) classification comprises N0 (no cancer-positive nodes), N1 (one or two cancer-positive nodes), N2 (three to six cancer-positive nodes), and N3 (seven or more cancer-positive nodes). The N classifications for cancers of the esophagus and esophagogastric junction are identical to the stomach cancer N classifications [7].
The M1a and M1b subclassifications have been eliminated, as has MX (Table 1). Distant metastasis is simply classified as M0, indicating no distant metastasis, and M1, indicating distant metastasis [7].
SEVENTH EDITION: NONANATOMIC CANCER CHARACTERISTICS
The nonanatomic classifications identified as important for stage grouping (Table 1) are the histopathologic cell type, histologic grade, and tumor location (Fig. 2). The difference in survival between adenocarcinoma and squamous cell carcinoma is best captured by including separate stage groupings for stages I and II. Increasing histologic grade is associated with incrementally decreasing survival for early-stage cancers. For adenocarcinoma, it is important to distinguish G1 and G2 (well differentiated and moderately differentiated) from G3 (poorly differentiated) for stage I and stage IIA cancers. For squamous cell carcinoma, distinguishing G1 from G2 and G3 is important for stage I and II cancers. Tumor location (upper and middle thoracic tumors versus lower thoracic tumors) is important for grouping T2-3N0M0 squamous cell cancers.
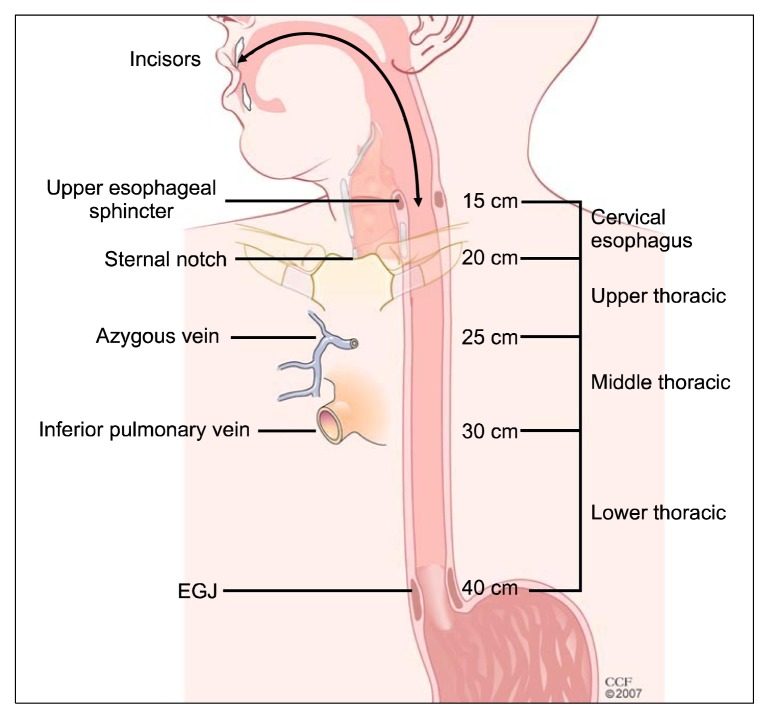
Fig. 2.
Cancer location. The cervical esophagus, bounded superiorly by the cricopharyngeus and inferiorly by the sternal notch, is typically 15–20 cm from the incisors using esophagoscopy. The upper thoracic esophagus, bounded superiorly by the sternal notch and inferiorly by the azygos arch, is typically 20–25 cm from the incisors using esophagoscopy. The middle thoracic esophagus, bounded superiorly by the azygos arch and inferiorly by the inferior pulmonary vein, is typically 25–30 cm from the incisors using esophagoscopy. The lower thoracic esophagus, bounded superiorly by the inferior pulmonary vein and inferiorly by the lower esophageal sphincter, is typically 30–40 cm from the incisors using esophagoscopy; this location includes cancers whose epicenter is within the proximal 5 cm of the stomach that extend into the EGJ or lower thoracic esophagus. EGJ, esophagogastric junction.
SEVENTH EDITION STAGE GROUPINGS
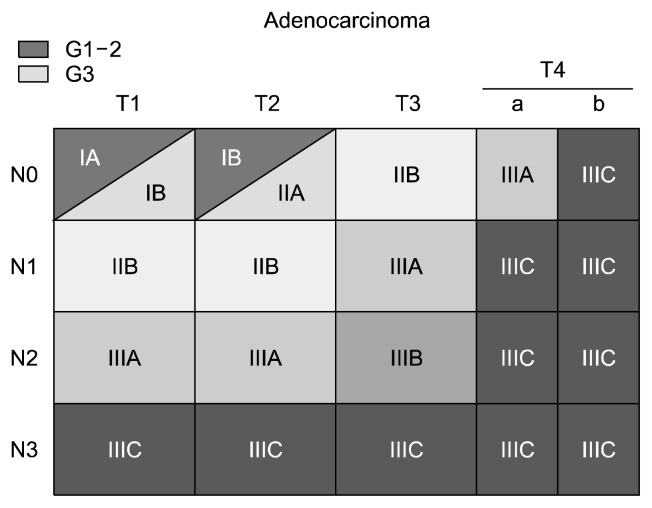
Stages 0 and IV are defined a prior as TisN0M0 and M1 tumors with any T and N stages, respectively. The stage groupings for M0 adenocarcinoma are shown in Fig. 3. T1N0M0 and T2N0M0 adenocarcinomas are subclassified by histologic grade: G1 and G2 are grouped into one sub-classification, while G3 tumors are grouped into a second subclassification.
Fig. 3.
Stage groupings for M0 adenocarcinoma by T and N classification and histologic grade (G).
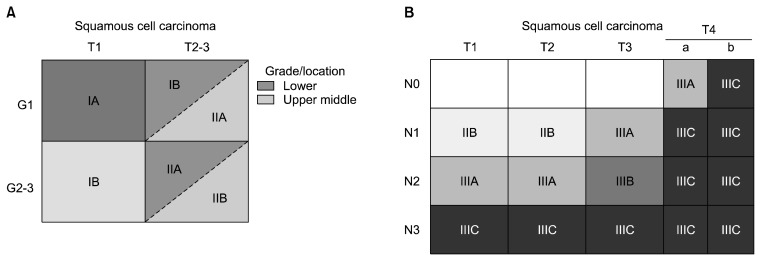
The stage groupings for M0 squamous cell carcinoma are shown in Fig. 4. T1N0M0 squamous cell carcinoma is sub-classified according to histologic grade: G1 tumors are opposed to G2 and G3 tumors (Fig. 4A). For T2N0M0 and T3N0M0 squamous cell carcinoma, the stage groupings follow histologic grade and location (Fig. 4A). The four combinations range from G1 lower thoracic squamous cell carcinoma (stage IB), which has the best survival, to G2–G4 upper and middle thoracic squamous cell carcinomas (stage IIB), which have the worst prognosis. G2–G4 lower thoracic squamous cell carcinomas and G1 upper and middle thoracic squamous cell carcinomas are grouped together (stage IIA), with an intermediate survival rate.
Fig. 4.
(A) Stage groupings for M0 squamous cell carcinoma. Stage groupings for T1N0M0 and T2-3N0M0 squamous cell carcinomas by histologic grade (G) and cancer location. (B) Stage groupings for M0 squamous cell carcinomas.
Stage 0, III, and IV adenocarcinomas (Fig. 3) and squamous cell carcinomas (Fig. 4B) are grouped into the same stage. Adenosquamous carcinomas are staged as squamous cell carcinomas.
The UICC has published empirical 7th edition TNM stage groupings in an attempt to preserve anatomic TNM staging, which ignores nonanatomic cancer characteristics and data-driven recommendations. These observational stage groupings should be used with care.
ESOPHAGOGASTRIC JUNCTION CANCERS
Besides being data-driven, the seventh edition of the Cancer Staging Manual harmonizes cancer staging across the esophagogastric junction. Previous editions produced different stages for these cancers depending on whether esophageal or stomach stage groupings were used. The seventh edition staging applies to cancers of the esophagus and esophagogastric junction, including cancers within the first 5 cm of the stomach that invade the esophagogastric junction.
SURVIVAL
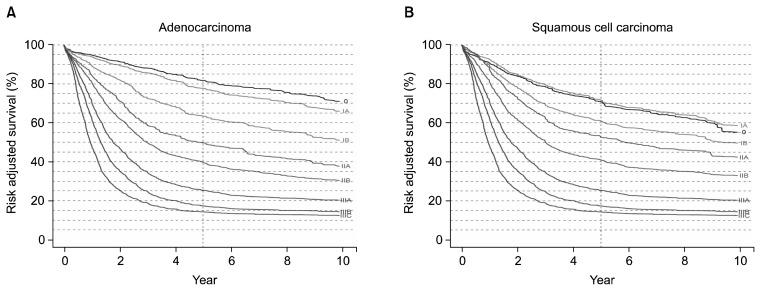
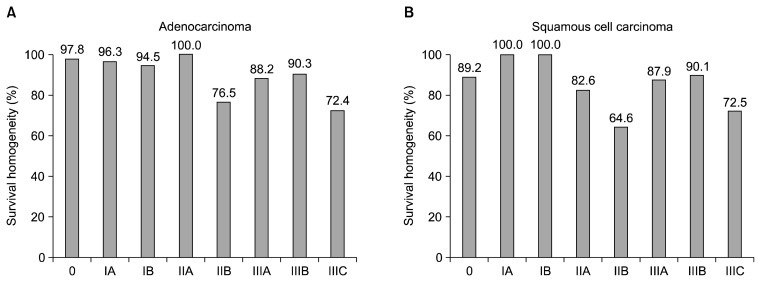
In the seventh edition classification, survival monotonically decreases with increasing stage groupings, and survival rates are markedly different among the groups (Fig. 5). However, due to rigid stage grouping definitions and limitations of the data, homogeneous survival rates are not seen in all groups (Fig. 6). Despite these shortcomings, the seventh edition represents a major evolutionary step in esophageal cancer staging. The risk-adjusted survival curves contained in the seventh edition can be used, with a recognition of their limitations, for crude prediction of the survival rate of various groups of patients.
Fig. 5.
(A) Risk-adjusted survival for adenocarcinoma according to the seventh edition American Joint Committee on Cancer/International Union Against Cancer stage groupings. (B) Risk-adjusted survival for squamous cell carcinoma according to the seventh edition American Joint Committee on Cancer/International Union Against Cancer stage groupings.
Fig. 6.
(A) Homogeneity of survival rates within the seventh edition stage groupings of adenocarcinoma of the esophagus. (B) Homogeneity of survival rates within the seventh edition stage groupings of squamous cell carcinoma of the esophagus.
THE FUTURE: THE EIGHTH EDITION AND BEYOND
The seventh edition heralded the era of data-driven cancer staging [8]. However, the seventh edition was derived from data that only reflected patients who underwent esophagectomy, which is an obvious shortcoming. The following steps will be necessary for improving the next iterations of esophageal cancer staging:
Obtaining better homogeneity within stage 0 and stage IV (Fig. 6). This will require abandoning the restrictive definitions of these stage groupings and changing the composition of the adjacent stage IA and stage IIIC groupings.
Improving the homogeneity of stage IIB adenocarcinoma (Fig. 6A) and stage IIA and IIB squamous cell cancer (Fig. 6B). This will require expanding the database of these less common cancers.
Adding clinical, post-induction clinical, post-definitive nonsurgical clinical, and post-induction pathologic staging recommendations. This will require expanding the data analysis.
Assessing other nonanatomic tumor characteristics that affect survival. This will require expanding the data to include factors beyond the histopathologic cell type, histologic grade, and cancer location.
Adding non-esophagectomy survival data, reflecting endoscopic treatment for stage 0 and stage IA tumors and palliative therapy for stage IV tumors. This will require partnering with non-surgical specialties and professional associations and groups.
Adding cancer of the cervical esophagus. This will require partnering and harmonizing with the head and neck task force, mirroring the process used with the gastric cancer task force in the seventh edition.
The acquisition of international multicenter data through the Worldwide Esophageal Cancer Collaboration is key to this effort [1]. Innovative machine learning techniques will again be used for analyzing the data [2]. The strategy for adding clinical, post-induction and definitive nonoperative therapy clinical, and post-induction pathologic staging will be to reference these stages to the pStaging platform of the eighth edition [9].
BEYOND ANATOMIC STAGING: TREATMENT DECISIONS AND PROGNOSIS
Differences in the focus and goals of the AJCC and UICC in cancer staging may be obviated by the extinction of the printed manual and the development of an internet cancer staging site. This will eliminate the need for a blanket change to all organ systems every six to seven years, permitting ongoing changes to each organ system when adjustments are necessary.
Stage groupings, although important for sets of patients, epidemiologists, and clinical situations where the gross characterization of clusters of patients is required, is an attempt to amalgamate data that eliminates significant information that may be crucial for an individual patient. The use of patient and treatment factors will be expanded in future analyses that will focus on the individual patient. Patient-specific prognosis requires that such factors be included as variables in the analyses instead being controlled for through risk adjustment, as was done in the seventh edition. The analyses will provide two models: a treatment decision model based on clinical staging and additional patient factors that will assist in treatment decisions, and a prognostic model based on pathologic staging, patient factors, and treatment delivered, which will facilitate prognostication [9]. Smartphone applications or the equivalent are envisioned for patient and physician use.
CONCLUSION
The concept of TNM cancer staging describing the anatomic extent of tumors was developed between 1943 and 1952. However, it was not applied to esophageal cancer until 1968 by the UICC and 1977 by the AJCC. Faithful adherence to the empiric staging process that was based on the stepwise progression of increasing local cancer invasion (T), followed by metastases to regional lymph nodes (N), and finally metastases to distant sites (M) dominated esophageal cancer staging for the next 33 years through six editions.
The staging recommendations for cancer of the esophagus and esophagogastric junction contained in the seventh edition are data-driven and have been harmonized with the staging of stomach cancer. Doing so required changing the TNM definitions and incorporating nonanatomic cancer characteristics. For cancers of the esophagus and esophagogastric junction, stages 0, III, and IV are identical for both adenocarcinoma and squamous cell carcinoma. However, stage groupings differ for stage I and II cancers based on histopathologic cell type, histologic grade, and cancer location.
Improving cancer staging requires moving beyond the strict TNM description of anatomic staging. The inclusion of TNM variables along with other variables that have yet to be identified will allow a more complete definition of esophageal tumors, aid in treatment decisions, and facilitate the accurate prediction of the prognosis of each patient.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Rice TW, Rusch VW, Apperson-Hansen C, et al. Worldwide esophageal cancer collaboration. Dis Esophagus. 2009;22:1–8. doi: 10.1111/j.1442-2050.2008.00901.x. [DOI] [PubMed] [Google Scholar]
- 2.Ishwaran H, Blackstone EH, Apperson-Hansen C, Rice TW. A novel approach to cancer staging: application to esophageal cancer. Biostatistics. 2009;10:603–20. doi: 10.1093/biostatistics/kxp016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rice TW, Rusch VW, Ishwaran H, Blackstone EH Worldwide Esophageal Cancer Collaboration. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer. 2010;116:3763–73. doi: 10.1002/cncr.25146. [DOI] [PubMed] [Google Scholar]
- 4.American Joint Committee on Cancer; American Cancer Society; American College of Surgeons. AJCC cancer staging manual. 7th ed. New York: Springer; 2010. [Google Scholar]
- 5.Sobin LH, Gospodarowicz MK, Wittekind C International Union against Cancer. TNM classification of malignant tumours. 7th ed. Chichester: Wiley-Blackwell; 2009. [Google Scholar]
- 6.Rice TW. Staging of esophageal cancer: TNM and beyond. Esophagus. 2010;7:189–95. doi: 10.1007/s10388-010-0246-4. [DOI] [Google Scholar]
- 7.Li Z, Rice TW. Diagnosis and staging of cancer of the esophagus and esophagogastric junction. Surg Clin North Am. 2012;92:1105–26. doi: 10.1016/j.suc.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Rusch VW, Rice TW, Crowley J, Blackstone EH, Rami-Porta R, Goldstraw P. The seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Staging Manuals: the new era of data-driven revisions. J Thorac Cardiovasc Surg. 2010;139:819–21. doi: 10.1016/j.jtcvs.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Rice TW, Blackstone EH. Esophageal cancer staging: past, present, and future. Thorac Surg Clin. 2013;23:461–9. doi: 10.1016/j.thorsurg.2013.07.004. [DOI] [PubMed] [Google Scholar]