Abstract
PURPOSE
This study was performed to assess the safety, efficacy, and clinical outcomes of transjugular intrahepatic portosystemic shunt (TIPS) creation for treatment of medically refractory as-cites and to identify prognostic factors for clinical response, morbidity, and mortality.
MATERIALS AND METHODS
In this retrospective study, 80 patients (male:female, 52:28; mean age, 56 years; mean Model for End-Stage Liver Disease [MELD] score, 15.1) who underwent elective TIPS creation for refractory ascites between 1999–2012 were studied. A medical record review was performed to identify data on demographics, liver disease, procedures, and outcome. The influence of these parameters on 30-day, 90-day, and one-year mortality was assessed using binary logistic regression. Overall survival was analyzed with Kaplan-Meier statistics.
RESULTS
TIPS was successfully created using covered (n=70) or bare metal (n=10) stents. Hemodynamic success was achieved in all cases. The mean final portosystemic pressure gradient (PSG) was 6.8 mmHg. Thirty-day complications included mild encephalopathy in 35% of patients. Clinical improvement in ascites occurred in 78% of patients, with complete resolution or a ≥50% decrease in 66% of patients. No predictors of response or optimal PSG threshold were identified. The 30-day, 90-day, and one-year mortality rates were 14%, 23%, and 33%, respectively. Patient age (P = 0.026) was associated with 30-day mortality, while final PSG was associated with 90-day (P = 0.020) and one year (P = 0.032) mortality. No predictors of overall survival were identified.
CONCLUSION
TIPS creation effectively treats medically refractory ascites with nearly 80% efficacy. The incidence of mild encephalopathy is nontrivial. Older age and final PSG are associated with mortality, and these factors should be considered in patient selection and procedure performance.
The development of medically refractory ascites is associated with a grave prognosis in patients with liver cirrhosis. One-year survival in this population is less than 50%, and there is an increased risk of complications such as spontaneous bacterial peritonitis, hepatorenal syndrome, and dilutional hyponatremia (1). Moreover, these patients typically have low Model for End-Stage Liver Disease (MELD) scores despite their high mortality rate, and thus hold low positions on national transplant listings (2, 3). Transjugular intrahepatic portosystemic shunt (TIPS) creation, an established treatment for complications of portal hypertension, has demonstrated utility in patients with refractory ascites (4). By diverting blood from the portal venous system to the systemic circulation, TIPS acts to lower hepatic sinusoidal pressure and increase effective circulatory flow, thereby reducing excess sodium retention and achieving ascites recurrence rates as low as 30% (5). Two recent studies revealed reduced mortality in patients undergoing TIPS placement, compared with those receiving serial large-volume paracentesis procedures, with one-year survival rates ranging from 63% to 80% (6, 7). Despite these objective benefits, adverse sequelae of TIPS, such as hepatic encephalopathy, may temper its utility, and predictive factors for clinical outcomes, such as ascites control, remain unclear (8). Mortality after TIPS creation has been associated with a variety of factors, including persistent refractory ascites, patient age, procedural urgency, various laboratory parameters, various liver disease scoring systems, and the occurrence of hepatic encephalopathy (7, 9–11). However, an ideal prognostic tool remains to be found.
While the benefits of TIPS creation for refractory ascites are well documented, the lingering inability to accurately predict adverse events and responses to treatment warrants further evaluation. Thus, this investigation was undertaken to review the safety, efficacy, and clinical outcomes of elective TIPS creation in a large single-center cohort of patients with refractory ascites and conduct, thereby, a detailed analysis of prognostic factors associated with clinical response, morbidity, and mortality.
Materials and methods
This retrospective study was conducted in compliance with the Health Insurance Portability and Accountability Act, and the institutional review board at our institution granted approval with a waiver of consent for inclusion in the study. All patients provided written informed consent for TIPS procedures, which were performed within the medical standard of care for treatment of medically refractory ascites.
Clinical setting and study design
Between November 1999 and July 2012, consecutive patients with liver cirrhosis who underwent successful TIPS creation at a single tertiary care, academic university-affiliated hospital situated in a large metropolitan area were identified and selected for this retrospective study. Patients were identified through a review of our hospital’s picture archiving and communication system.
Patients and liver disease
A total of 246 patients who underwent technically successful TIPS creation were identified for potential inclusion in this retrospective study. Of these, patients who underwent elective TIPS creation for treatment of medically refractory ascites were selected for analysis. Those patients lacking clinical data detailing the number of paracentesis procedures within 90 days prior to and following TIPS creation were excluded from analysis because of the inability to assess TIPS efficacy (n=2). Thus, 80 patients were included in the final study cohort. Patient demographics and liver disease characteristics of the study cohort are summarized in Table 1. Medically refractory ascites was not adequately controlled by conventional therapy, including dietary sodium restriction, fluid restriction, and diuretic therapy. Previously transplanted patients comprised six (7.5%) of 80 patients in the study cohort.
Table 1.
Patient demographics and liver disease characteristics
| Mean±SD or n (%) n=80 | |
|---|---|
| Age (years) | 55.8±8.0 |
| Male gender | 52 (65) |
| Liver disease etiology | |
| Alcohol | 21 (26) |
| HBV or HCV | 25 (31) |
| Alcohol and HBV or HCV | 23 (29) |
| Othera | 11 (14) |
| MELD score | 15.1±5.4 |
| MELD-Na score | 19.1±5.4 |
| Child-Pugh score | 9.8±1.6 |
| A | 0 |
| B | 46 (58) |
| C | 34 (42) |
Includes nonalcoholic steatohepatitis, primary biliary cirrhosis, cryptogenic liver disease, autoimmune liver disease, alpha-one antitrypsin deficiency, congenital hepatic fibrosis, idiopathic adult ductopenia, and unknown causes of cirrhosis.
HBV, hepatitis B virus; HCV, hepatitis C virus; MELD, Model for End-Stage Liver Disease; SD, standard deviation.
TIPS procedures
The technique for TIPS placement has been previously described (12). Procedures were performed in the Interventional Radiology suite using general anesthesia. Intravenous antibiotics were administered prior to the procedure. Right jugular venous access was gained with dilation to the diameter of a 10 F sheath. A 5 F catheter was used to engage the right hepatic vein. After hepatic venography and pressure measurement, wedged hepatic venography was performed. Next, a Rösch-Uchida transjugular liver access set (Cook Medical Co., Bloomington, Indiana, USA) was used to access the right portal vein. After portal vein catheterization and direct portal vein pressure measurement, balloon dilation of the hepatic parenchymal tract was performed. Next, direct portography was performed. Subsequently, 10-or 12-mm Wallstent bare metal stents (Boston Scientific, Natick, Massachusetts, USA) (used between 1999–2003) or 10-mm covered stent grafts (W.L. Gore & Associates, Flagstaff, Arizona, USA) (used between 2004–2012) were deployed across the liver tract. If the distal shunt fell short of the hepatic vein to the inferior vena cava junction, additional stents were utilized to extend the shunt. Balloon angioplasty was performed using a 7- to 10-mm balloon. After measurement of the final portal and right atrial pressures, shunt venography was performed.
Postprocedure care and clinical follow-up
Following the TIPS procedures, patients were monitored in the intensive care unit. Immediate postprocedure clinical follow-up was performed while patients remained hospitalized following TIPS creation. Subsequent clinical follow-up took place in the outpatient hepatology clinic. All patients underwent TIPS surveillance; those who had undergone bare metal stent TIPS procedures were subjected to direct shunt venography at one-, three- and six-month postprocedural intervals, and those who had undergone covered stent TIPS procedures were subjected to color Doppler ultrasound imaging at one-, three- and six-month postprocedural intervals.
Measured outcomes
The outcomes measured in this study included TIPS hemodynamic success, safety, ascites control, and patient mortality. The effects of demographic factors, liver disease scores, and procedure parameters on patient clinical outcomes, including development of new or worsening hepatic encephalopathy, ascites control, and patient survival, were investigated. TIPS hemodynamic success was defined as reduction in the portosystemic pressure gradient (PSG) to an absolute value of <12 mmHg. The final PSG was measured from the portal vein to the right atrium, which is a widely used and supported method (13). Safety was measured through identification of procedure-related complications, which were classified according to the Society of Interventional Radiology Standards of Practice Committee classification of complications (14). Post-TIPS hepatic encephalopathy was defined as the development of new or worsening mental status changes (confusion) or alterations in the level of consciousness. Presence of hepatic encephalopathy was clinically determined by the patient’s hepatologist and graded according to the West Haven classification system (15). Ascites control was assessed by comparing paracentesis frequency within 90 days prior to and following TIPS creation. Patient transplant-free survival was evaluated at 30 days, 90 days, and one year after TIPS. Overall transplant-free survival was also evaluated. Patient mortality was identified through electronic medical record review and confirmed using the United States Social Security Death Index.
Statistical analysis
Descriptive statistics were used to check for erroneous entries, assess for normalcy of the data, and characterize the demographic features of the study population. Comparisons of continuous normally distributed variables were performed by the independent-samples t test. Pre- and post-TIPS paracentesis frequency was compared using the paired samples t test. Comparisons of categorical data were performed using Pearson’s chi-squared test. Multivariate binary logistic regression analysis was used to assess the influence of demographic factors, liver disease scores, and procedure parameters on patient clinical outcomes, including occurrence of hepatic encephalopathy, ascites control, and transplant-free survival at 30 days, 90 days, and one year. A significance level of P ≤ 0.10 in univariate analysis was used as a cutoff to include a variable in multivariate analysis. Patient overall transplant-free survival was assessed using Kaplan-Meier statistics with log-rank comparison to determine prognostic factors for long-term survival. Variables with a significance level of P ≤ 0.10 in univariate Kaplan-Meier analysis were included in multivariate analysis using the Cox proportional hazards test. Statistical analyses were performed with a commercially available software package (SPSS version 18, SPSS Inc., Chicago, Illinois, USA). P values of ≤ 0.05 were considered statistically significant.
Results
TIPS procedures and procedure-related complications
TIPS procedure results are summarized in Table 2. TIPS was created with covered stent grafts in 70 of 80 patients (88%) and bare metal stents in 10 (12%) of 80 patients. Thirty-day procedure-related adverse events included hepatic encephalopathy in 28 (35%) of 80 patients, hepatic decompensation in two (2.5%) of 80 patients, and liver insufficiency requiring shunt reduction in one (1%) of 80 patients. Of note, encephalopathy was predominantly minimal or mild, with 26 (93%) of 28 patients categorized as grade 0 or 1, and two (7%) of 28 patients classified as grade 2. Univariate analysis identified no demographic or procedural parameters associated with the occurrence of hepatic encephalopathy.
Table 2.
TIPS results
| Mean±SD or n (%) n=80 | |
|---|---|
| Hemodynamic success | 80 (100) |
| Baseline RA pressure (mmHg)a | 12.2±4.5 |
| Final RA pressure (mmHg)b | 17.5±6.0 |
| RA pressure increase (mmHg) | 5.4±4.1 |
| Pre-TIPS PSG (mmHg)c | 20.4±6.2 |
| Post-TIPS PSG (mmHg) | 6.8±2.3 |
| PSG reduction (mmHg) | 13.7±6.3 |
Baseline RA pressure missing in 15 patients.
Final RA pressure missing in 33 patients.
Pre-TIPS PSG values missing in two patients.
PSG, portosystemic gradient; RA, right atrial; SD, standard deviation; TIPS, transjugular intrahepatic portosystemic shunt.
Ascites control
During the three months prior to TIPS creation, patients underwent a mean of 6.4±4.7 paracentesis procedures. In the three months after TIPS creation, there was a statistically significant reduction in the number of paracentesis procedures to 2.3±3.0 (P < 0.001). At 90 days post-TIPS creation, clinical improvement in ascites was evident in 78% of patients (n=62, Fig. 1). Complete resolution of ascites requiring no additional paracentesis procedures occurred in 35% (n=28), while the frequency of paracentesis procedures was reduced by ≥50% in 31% (n=25) and by <50% in 11% of patients (n=9). No change in paracentesis frequency occurred in 11% of patients (n=9), and another 11% (n=9) required more frequent paracentesis procedures after TIPS creation.
Figure 1.
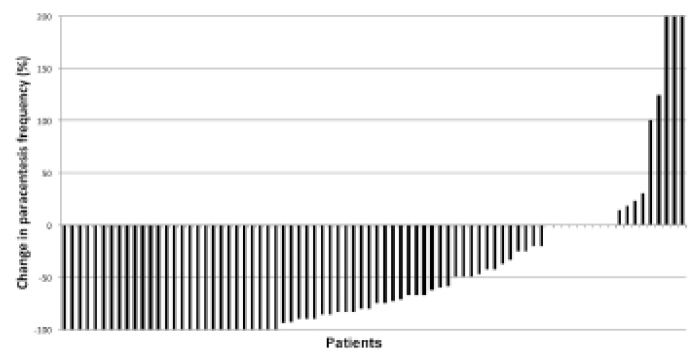
Waterfall plot of individual patient responses to transjugular intrahepatic portosystemic shunt (TIPS) creation for treatment of refractory ascites. The rates of complete and partial responses to TIPS creation were 35% and 43%, respectively. The rate of stable or progressive paracentesis was 22%.
Univariate analysis revealed that the mean international normalized ratio (INR) was significantly higher in the unimproved group (1.8 vs. 1.4, P = 0.008), but this was not significant on multivariate binary logistic regression analysis. Baseline characteristics were otherwise similar between the improved and unimproved groups, as were the mean initial (20.3±6.3 vs. 21.0±5.7 mmHg, P = 0.657) and final (6.8±2.3 vs. 6.8±2.3 mm Hg, P = 0.986) PSG and the PSG reduction (13.5±6.3 vs. 14.4±6.3 mmHg, P = 0.591). There was also no difference between percent PSG reduction between improved and unimproved groups (−65±14% vs. −67±11%, respectively, P = 0.605). No optimal PSG threshold for ascites control was identified; the final PSG in the improved group ranged from 2 to 11 mmHg, and the final PSG in the unimproved group ranged from 1 to 11 mmHg (Fig. 2). At the PSG threshold of 8 mmHg recommended by the American Association for the Study of Liver Diseases (AASLD) for treatment of refractory ascites (16), 48 (79%) of 61 patients achieved clinical improvement in ascites with reduced paracentesis frequency. In comparison, the rates of clinical improvement for the 12 mmHg (62 of 80 patients, 78%) and 10 mmHg (61 of 77 patients, 79%) thresholds showed no statistically significant differences in clinical response (P = 0.965).
Figure 2.
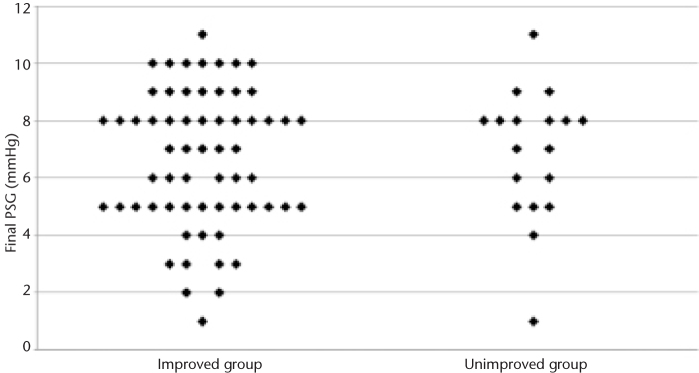
Plot of final portosystemic pressure gradients in the improved and unimproved groups reveals no optimal threshold for ascites control.
Transplant-free patient survival
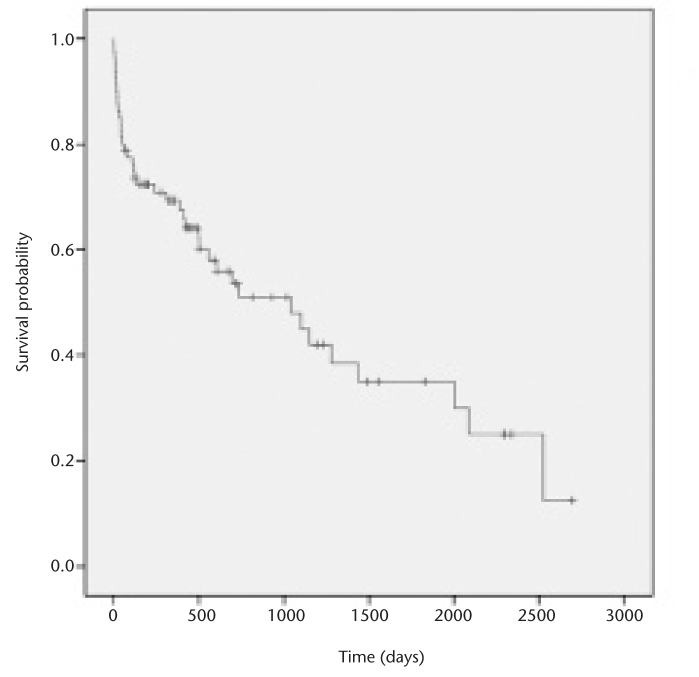
The patient follow-up time ranged from two to 2696 days. In total, 9% of the study cohort (n=7) underwent liver transplantation within one year after TIPS creation (median, 197±71 days post-TIPS). The median survival in the study cohort was 1044 days (range, 2–2696 days). The overall 30-day, 90-day, and one-year patient mortality rates were 14% (n=11), 23% (n=18), and 33% (24 of 73 patients). Kaplan-Meier survival analysis results of the study cohort are shown in Fig. 3. Causes of death within one year included liver and/or multiorgan failure (n=4), septic shock (n=6), respiratory failure (n=2), intraperitoneal hemorrhage (n=1), meningitis (n=1), and not specified (n=10).
Figure 3.
Kaplan-Meier estimation of survival probability among patients undergoing transjugular intrahepatic portosystemic shunt creation for refractory ascites demonstrates 87%, 77%, and 72% for 30-day, 90-day, and one-year transplant-free survival, respectively.
Prognostic factors for survival
At 30 days, univariate analysis showed that the bilirubin level (1.7 vs. 2.3 mg/dL, P = 0.100), platelet count (123.5 vs. 77.2×103/mL, P = 0.092), age (55.2 vs. 59.5 years, P = 0.100), MELD score (14.6 vs. 18.1, P = 0.045), and MELD-Na score (18.6 vs. 22.5, P = 0.026) were associated with mortality. Binary logistic regression analysis confirmed a statistically significant association between patient age and 30-day mortality (P = 0.026). Thirty-day mortality was 12% (seven of 57 patients) in patients aged ≤59 years and 17% (four of 23 patients) in those aged ≥60 years.
At 90 days, univariate analysis showed that the INR (1.4 vs. 1.9, P = 0.001), Child-Pugh score (9.6 vs. 10.3, P = 0.089), MELD score (14.1 vs. 18.5, P = 0.002), MELD-Na score (18.0 vs. 22.8, P = 0.001), and final PSG (7.3 vs. 5.3 mmHg, P = 0.001) were associated with mortality. Binary logistic regression analysis identified a statistically significant association between final PSG (P = 0.020) and 90-day mortality.
At one year, univariate analysis revealed that the INR (1.3 vs. 1.8, P < 0.001), bilirubin level (1.4 vs. 2.4 mg/dL, P < 0.001), platelet count (135 vs. 88×103/mL, P = 0.031), MELD score (13.2 vs. 18.2, P < 0.001), MELD-Na score (17.3 vs. 22.4, P < 0.001), Child-Pugh score (9.4 vs. 10.5, P = 0.005), and final PSG (7.4 vs 5.5 mm Hg, P = 0.001) were associated with mortality. The final PSG proved significant on binary logistic regression (P = 0.032).
Univariate analysis of Kaplan-Meier overall survival identified MELD score (<18 vs. >18; median survival, 1100 vs. 130 days; P = 0.095), final PSG (≥8 vs. <8; median survival, 1280 vs. 503 days; P = 0.047), and gender (male vs. female; median survival, 1437 vs. 500 days; P = 0.032) as prognostic factors for long-term survival. None of these parameters were found to be statistically significant on Cox regression analysis.
PSG and survival outcomes
In patients with a final PSG of <5 mmHg, the 90-day mortality rate was 46% (five of 11 patients) compared with 19% (13 of 69 patients) in those with a PSG of ≥5 mmHg (P = 0.112). At the AASLD-recommended PSG threshold of 8 mmHg, 90-day mortality was 33% (14 of 42 patients) when the PSG was <8 mmHg compared with 11% (four of 38 patients) in those with a PSG of ≥8 mmHg (P = 0.015). One-year mortality was 45% (18 of 40 patients) when the final PSG was <8 mmHg and 18% (six of 33 patients) with a PSG of ≥8 mmHg.
TIPS revisions
A total of 26 TIPS revisions were performed in 20 (25%) of 80 patients. The revision rates in the bare metal stent TIPS and covered stent TIPS cohorts were 40% (four of 10 patients) and 24% (17 of 70 patients), respectively. Indications for revision included recurrent or persistent clinical symptoms (n=20), ultrasound abnormalities (n=5), and intervention during scheduled follow-up venography (n=1). Venographic findings included shunt stenosis (n=10), occlusion (n=2), thrombus (n=4), and occult PSG elevation or PSG elevation to ≥12 mmHg with no venographic abnormalities (n=10). Interventions comprised angioplasty (n=15), shunt extension or relining (n=4), and angioplasty in conjunction with shunt extension or relining (n=7).
Discussion
In this investigation, TIPS were created with high technical and hemodynamic success in a large cohort of patients with medically refractory ascites. Clinical improvement occurred in 78% of patients, 35% of whom experienced complete resolution of their ascites. These results compare favorably with those of other large series in which the efficacy rates ranged from 38% to 84%, further affirming the utility of TIPS in this setting (17). Prediction of the clinical response remains difficult, however. While the Child-Pugh score, MELD-Na score, platelet count, and INR were significantly associated with a treatment response on univariate analysis, none proved significant upon multivariate analysis. Furthermore, an optimal PSG level for ascites control was not apparent, with rates of improvement and unimprovement occurring across similar final PSG ranges (1–11 or 2–11 mmHg) and thresholds (8, 10, and 12 mmHg). Receiver operator characteristic curve analysis (data not shown) did not identify PSG thresholds that reliably predicted improvement from unimprovement after TIPS creation with high sensitivity or specificity.
Despite the considerable efficacy of TIPS in resolving refractory ascites, the severity of the disease may, in some cases, preclude resolution or improvement. In these patients, optimal control may require a degree of PSG reduction that would entail a prohibitively high risk of liver decompensation and mortality. While the AASLD recommends a final PSG target of 8 mmHg (16), our results indicate that a final PSG of 12 mmHg, similar to that aimed at in cases of variceal hemorrhage, may be adequate. A higher PSG reduces the risk for low-flow liver complications by reducing portosystemic shunting and increasing perfusion of native portal vessels (18).
The morbidity rates noted herein were comparable with established figures, confirming the relatively high risk of hepatic encephalopathy following TIPS creation (19, 20). Unfortunately, final PSG levels that may improve medically refractory ascites also increase the risk of hepatic encephalopathy (21, 22). Although we found no independent predictors of hepatic encephalopathy, it should be noted that nearly all instances of hepatic encephalopathy were mild cases, graded at 0 or 1, which are generally treatable with dietary modification and medical therapy (23–25). Importantly, the occurrence of encephalopathy was not significantly associated with mortality in our cohort. To this end, hepatic encephalopathy should not be considered a significant contraindication to TIPS in the setting of medically refractory ascites, particularly if a proactive approach to prevention with empiric medical therapy is pursued.
With regard to TIPS mortality, we found that survival at 90 days and one year was significantly associated with post-TIPS PSG reduction. Patients with refractory ascites are typically characterized by cirrhotic liver disease and are vulnerable to hepatic decompensation following excess shunting of blood away from the liver (24). A low post-TIPS PSG predicted early mortality in our cohort; patients with a final PSG of <8 mmHg, as recommended by the AASLD, had a 33% mortality rate at 90 days in contrast to 11% in those with a PSG of ≥8 mmHg. One-year mortality was 45% (18 of 40 patients) in those with a PSG of <8 mmHg and 18% (six of 33 patients) in those with a PSG of ≥8 mmHg. This finding echoes a recent study in which PSG reduction below 8 mmHg was associated with a three-fold increase in the likelihood of mortality (26). In examining the lower threshold for PSG reduction during TIPS creation, Chung et al. (27) found that a level of 5 mmHg is associated with medical complications. Our results also corroborate this finding: patients in our cohort with a final PSG of <5 mmHg had a 46% (five of 11 patients) 90-day and 64% (seven of 11 patients) one-year mortality compared with 19% (13 of 69 patients) and 27% (17 of 62 patients) in those with a PSG of ≥5 mmHg. This threshold may represent a limit beyond which the risks of aggressive intervention supersede the benefits of ascites reduction.
Mortality at one month was 14%; this nontrivial incidence highlights the fact that TIPS creation, although technically safe, contributes to physiologic alterations that may ultimately account for increased mortality in patients with severe underlying liver disease. This may be of particular relevance in patients of advanced age, who were significantly associated with 30-day mortality in the present study. The impact of age on survival has been previously described and is readily accounted for by the diminished functional hepatic reserve and increased burden of comorbidities among the elderly (26). These findings suggest that TIPS placement for refractory ascites should be pursued cautiously in older patients.
There are several limitations to this investigation. First, this study was retrospective and nonrandomized in nature and it is subject to the inherent weaknesses of nonprospective studies. Second, due to the retrospective nature of this study, not all data points were available for analysis; for example, we were unable to collect right atrial pressures for a number of patients in our cohort. Third, our investigation represents the experience of a single institution. Fourth, because patients in this study were included over a decade-long period, technical differences in TIPS placement and improvements in medical care during the study period may have contributed to differences in clinical outcomes over time. Fifth, our analysis of survival included deaths due to all causes rather than due to liver disease only.
In conclusion, TIPS creation is a safe and effective treatment for medically refractory ascites, eliminating or reducing the need for serial paracentesis procedures in almost 80% of cases within three months. While the rate of hepatic encephalopathy is nontrivial, most cases were mild, and our results further validate TIPS creation in this setting. Patient age and a low final PSG were associated with early- and intermediate-term mortality, and consideration of these factors may enhance patient selection and risk stratification in this population. Unfortunately, prognostic factors for clinical response remain unclear, highlighting the need for continued investigation to refine the optimal target PSG threshold.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397–417. doi: 10.1016/j.jhep.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Heuman DM, Abou-Assi SG, Habib A, et al. Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death. Hepatology. 2004;40:802–810. doi: 10.1002/hep.20405. [DOI] [PubMed] [Google Scholar]
- 3.Guy J, Somsouk M, Shiboski S, Kerlan R, Inadomi JM, Biggins SW. New model for end stage liver disease improves prognostic capability after transjugular intrahepatic portosystemic shunt. Clin Gastroenterol Hepatol. 2009;7:1236–1240. doi: 10.1016/j.cgh.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colombato L. The role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension. J Clin Gastroenterol. 2007;41(Suppl 3):S344–351. doi: 10.1097/MCG.0b013e318157e500. [DOI] [PubMed] [Google Scholar]
- 5.Saab S, Nieto JM, Lewis SK, Runyon BA. TIPS versus paracentesis for cirrhotic patients with refractory ascites. Cochrane Database Syst Rev. 2006:CD004889. doi: 10.1002/14651858.CD004889.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narahara Y, Kanazawa H, Fukuda T, et al. Transjugular intrahepatic portosystemic shunt versus paracentesis plus albumin in patients with refractory ascites who have good hepatic and renal function: a prospective randomized trial. J Gastroenterol. 2011;46:78–85. doi: 10.1007/s00535-010-0282-9. [DOI] [PubMed] [Google Scholar]
- 7.Salerno F, Camma C, Enea M, Rossle M, Wong F. Transjugular intrahepatic portosystemic shunt for refractory ascites: a meta-analysis of individual patient data. Gastroenterology. 2007;133:825–834. doi: 10.1053/j.gastro.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Thuluvath PJ, Bal JS, Mitchell S, Lund G, Venbrux A. TIPS for management of refractory ascites: response and survival are both unpredictable. Dig Dis Sci. 2003;48:542–550. doi: 10.1023/a:1022544917898. [DOI] [PubMed] [Google Scholar]
- 9.Masson S, Mardini HA, Rose JD, Record CO. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunt insertion: a decade of experience. QJM. 2008;101:493–501. doi: 10.1093/qjmed/hcn037. [DOI] [PubMed] [Google Scholar]
- 10.Bureau C, Metivier S, D’Amico M, et al. Serum bilirubin and platelet count: a simple predictive model for survival in patients with refractory ascites treated by TIPS. J Hepatol. 2011;54:901–907. doi: 10.1016/j.jhep.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 11.Heinzow HS, Lenz P, Kohler M, et al. Clinical outcome and predictors of survival after TIPS insertion in patients with liver cirrhosis. World J Gastroenterol. 2012;18:5211–5218. doi: 10.3748/wjg.v18.i37.5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaba RC, Omene BO, Podczerwinski ES, et al. TIPS for treatment of variceal hemorrhage: clinical outcomes in 128 patients at a single institution over a 12-year period. J Vasc Interv Radiol. 2012;23:227–235. doi: 10.1016/j.jvir.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Haskal ZJ, Martin L, Cardella JF, et al. Quality improvement guidelines for transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol. 2003;14:S265–270. [PubMed] [Google Scholar]
- 14.Brown DB, Cardella JF, Sacks D, et al. Quality improvement guidelines for transhepatic arterial chemoembolization, embolization, and chemotherapeutic infusion for hepatic malignancy. J Vasc Interv Radiol. 2006;17:225–232. doi: 10.1097/01.RVI.0000195330.47954.48. [DOI] [PubMed] [Google Scholar]
- 15.Blei AT, Cordoba J. Hepatic encephalopathy. Am J Gastroenterol. 2001;96:1968–1976. doi: 10.1111/j.1572-0241.2001.03964.x. [DOI] [PubMed] [Google Scholar]
- 16.Boyer TD, Haskal ZJ. American Association for the Study of Liver Diseases Practice Guidelines: the role of transjugular intrahepatic portosystemic shunt creation in the management of portal hypertension. J Vasc Interv Radiol. 2005;16:615–629. doi: 10.1097/01.RVI.0000157297.91510.21. [DOI] [PubMed] [Google Scholar]
- 17.Fidelman N, Kwan SW, LaBerge JM, Gordon RL, Ring EJ, Kerlan RK., Jr The transjugular intrahepatic portosystemic shunt: an update. AJR Am J Roentgenol. 2012;199:746–755. doi: 10.2214/AJR.12.9101. [DOI] [PubMed] [Google Scholar]
- 18.Walser EM, Harris VM, Harman JT, Park HM, Siddiqui AR. Quantification of intrahepatic portosystemic shunting after placement of a transjugular intrahepatic portosystemic shunt. J Vasc Interv Radiol. 1996;7:263–267. doi: 10.1016/s1051-0443(96)70775-3. [DOI] [PubMed] [Google Scholar]
- 19.Riggio O, Angeloni S, Salvatori FM, et al. Incidence, natural history, and risk factors of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt with polytetrafluoroethylene-covered stent grafts. Am J Gastroenterol. 2008;103:2738–2746. doi: 10.1111/j.1572-0241.2008.02102.x. [DOI] [PubMed] [Google Scholar]
- 20.Riggio O, Nardelli S, Moscucci F, Pasquale C, Ridola L, Merli M. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Clin Liver Dis. 2012;16:133–146. doi: 10.1016/j.cld.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Rossi P, Salvatori FM, Fanelli F, et al. Polytetrafluoroethylene-covered nitinol stent-graft for transjugular intrahepatic portosystemic shunt creation: 3-year experience. Radiology. 2004;231:820–830. doi: 10.1148/radiol.2313030349. [DOI] [PubMed] [Google Scholar]
- 22.Riggio O, Merli M, Pedretti G, et al. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Incidence and risk factors. Dig Dis Sci. 1996;41:578–584. doi: 10.1007/BF02282344. [DOI] [PubMed] [Google Scholar]
- 23.Zuckerman DA, Darcy MD, Bocchini TP, Hildebolt CF. Encephalopathy after transjugular intrahepatic portosystemic shunting: analysis of incidence and potential risk factors. AJR Am J Roentgenol. 1997;169:1727–1731. doi: 10.2214/ajr.169.6.9393198. [DOI] [PubMed] [Google Scholar]
- 24.Riggio O, Masini A, Efrati C, et al. Pharmacological prophylaxis of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt: a randomized controlled study. J Hepatol. 2005;42:674–679. doi: 10.1016/j.jhep.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 25.Wright G, Chattree A, Jalan R. Management of hepatic encephalopathy. Int J Hepatol. 2011;2011:841407. doi: 10.4061/2011/841407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan JJ, Chen C, Caridi JG, et al. Factors predicting survival after transjugular intrahepatic portosystemic shunt creation: 15 years’ experience from a single tertiary medical center. J Vasc Interv Radiol. 2008;19:1576–1581. doi: 10.1016/j.jvir.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 27.Chung HH, Razavi MK, Sze DY, et al. Portosystemic pressure gradient during transjugular intrahepatic portosystemic shunt with Viatorr stent graft: what is the critical low threshold to avoid medically uncontrolled low pressure gradient related complications? J Gastroenterol Hepatol. 2008;23:95–101. doi: 10.1111/j.1440-1746.2006.04697.x. [DOI] [PubMed] [Google Scholar]