Abstract
The VX2 tumor is a leporine anaplastic squamous cell carcinoma characterized by rapid growth, hypervascularity, and facile propagation in the skeletal muscle. Since its introduction over 70 years ago, it has been used to model a variety of malignancies, and is commonly employed by interventional radiologists in preclinical investigations of hepatocellular carcinoma. However, despite the widespread and lasting popularity of the model, there are few technical resources detailing its use. Herein, we present a comprehensive pictorial outline of the technical methodology for development, growth, propagation, and angiographic utilization of the rabbit VX2 liver tumor model.
The rabbit VX2 tumor model has played a longstanding role in experimental oncology. Developed in 1930–1940 by Rous et al. (1, 2), the VX2 tumor is a virus-induced anaplastic squamous cell carcinoma characterized by hypervascularity, rapid growth, and easy propagation in the skeletal muscle (3, 4). Since its introduction, the tumor has been used to model cancers of the head and neck (5), kidney (6), brain (7), lung (8), urinary bladder (9), uterus (10), liver (11, 12), bone (13), and pancreas (14). The high growth rate and the relatively large size of rabbit vasculature render the model particularly well suited for use by interventional radiologists, and in recent years the model has been employed in numerous studies pertaining to the imaging and locoregional treatment of hepatocellular carcinoma (15–19). However, despite the widespread and lasting popularity of the model, there are few, if any, comprehensive technical resources detailing its use, leaving many key procedural details to be conveyed anecdotally. Lack of a technical guide may also represent a barrier to entry of interventional radiologists into translational research. With that in mind, this review is intended to provide a complete pictorial overview of the development, growth, propagation, and angiographic utilization of the rabbit VX2 tumor model based on the experience of a single operator in order to serve as a reference for novice and experienced investigators alike.
Animal subjects and laboratory setting
The Institutional Animal Care and Use Committee approved the use of the animal subjects depicted in this review. New Zealand White rabbits weighing approximately 2.5–3.5 kg were used, and trained veterinary staff supported daily maintenance, pre-, peri-, and postprocedural care, procedural anesthesia, and sacrifice of the animals. All procedures were performed in a sterile environment.
VX2 hind limb tumor development
In the United States, the VX2 tumor cell line is available for purchase from the National Cancer Institute Division of Cancer Treatment Diagnosis and Treatment Tumor/Cell Line Repository (20). For tumor development, frozen VX2 sample is thawed in lukewarm water, aspirated into a 5–10 mL syringe, and mixed with an equal part of methylcellulose medium (Stemcell Technologies, Vancouver, British Columbia, Canada) (Fig. 1). A hind limb donor rabbit is sedated prior to inoculation with 1 mg/kg acepromazine and 0.02 mg/kg buprenorphine intramuscular injection; analgesia using meloxicam 0.2 mg/kg subcutaneous injection may also be provided. The inoculation site is shaved and cleaned with an alcohol- or iodine-based agent, and a 16-gauge needle is then used to inject the cell suspension into the gluteal muscles of the hind limb (Fig. 2). Approximately 1 mL of VX2 suspension is injected into each hind limb. Although initial tumor development requires use of frozen VX2 sample, use of a fresh VX2 inoculum should be prioritized for subsequent tumor propagation. In a study by Virmani et al. (21), inoculation of hind limb donor rabbits using fresh VX2 resulted in successful tumor growth in 88% of subjects, compared with 33% of subjects inoculated with frozen samples. Following inoculation, rabbits are monitored for appetite. Based on investigator experience, animals undergoing hind limb injection tend to lose appetite for food at approximately two weeks postinoculation. Other signs, such as tumor impairment of ambulation and tumor ulceration, are uncommon.
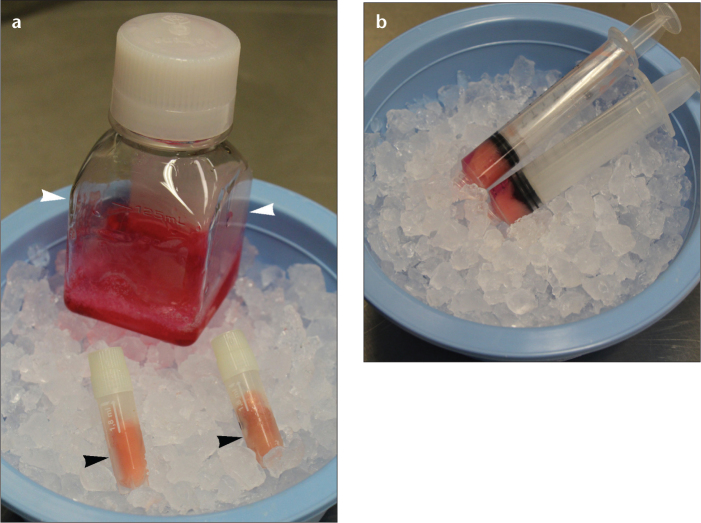
Figure 1. a, b.
Photograph (a) shows material required for VX2 hind limb tumor development, including frozen VX2 (black arrowheads) and methylcellulose medium (white arrowheads). Photograph (b) exhibits VX2 cells mixed in a 1:1 volumetric ratio with methylcellulose medium and kept on ice prior to inoculation.
Figure 2. a, b.
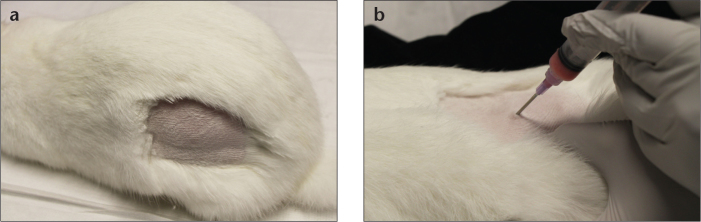
Donor rabbit inoculation. Photograph (a) depicts hind limb shaved and disinfected with alcohol or iodine preparation. Photograph (b) shows 16-gauge needle used for intramuscular injection of 1 mL VX2 mixture. Care is taken to avoid sciatic nerve, which runs along a palpable groove over the femur.
VX2 hind limb tumor growth and harvesting
A viable tumor will yield a palpable nodule within 2–3 weeks of inoculation (Fig. 3a). Signs of tumor growth in the donor rabbit may include decreased appetite, loss of caecotrophy (the ingestion of nocturnal feces is common to lagomorphs) and the development of lethargy and fever with highly necrotic tumors. Ultrasonography may also be used to confirm tumor growth (Fig. 3b). In cases of tumor nongrowth, repeat hind limb inoculation should be performed.
Figure 3. a, b.
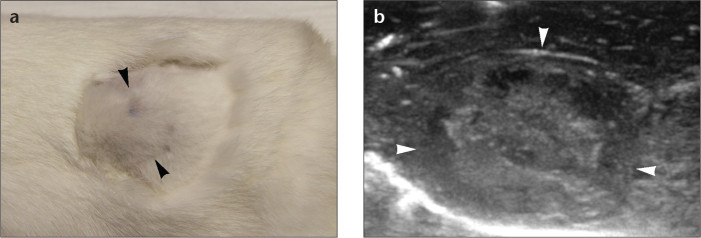
Photograph (a) reveals palpable subcutaneous hind limb nodule (arrowheads) indicating tumor growth two weeks after inoculation. Ultrasound image (b) shows large tumor implant (arrowheads).
Once tumor growth is established, hind limb tumor extraction may be pursued for tumor line propagation and liver tumor creation. The hind limb donor rabbit is medicated with intramuscular injections of 15–30 mg/kg ketamine and 50–250 µg/kg dexmeditomidine, and sacrificed using an intravenous dose of sodium pentobarbital exceeding 390 mg/kg. The cutaneous and subcutaneous tissues overlying the tumor nodule are divided and the hind limb muscle is exposed (Fig. 4a). Two curvilinear incisions are made within the muscle in order to remove the tumor nodule en-bloc (Fig. 4b). The explanted specimen is then bisected, permitting visualization of the necrotic core and peripheral viable tumor (Fig. 4c). Gentle scraping with a blunt instrument easily removes the necrotic core and allows for careful separation of viable tumor from adherent muscle tissue. Several pieces of tumor approximating 1–2 mm3 in size should be selected and stored in sterile saline for immediate subsequent intrahepatic implantation. The remaining tumor is bathed in nutrient-rich Roswell Park Memorial Institute (RPMI) medium (Sigma-Aldrich, St. Louis, Missouri, USA) and kept on ice for short-term storage until later processing (Fig. 4d). Kept cold in this manner, tumors may be preserved for handling up to 2–3 hours later.
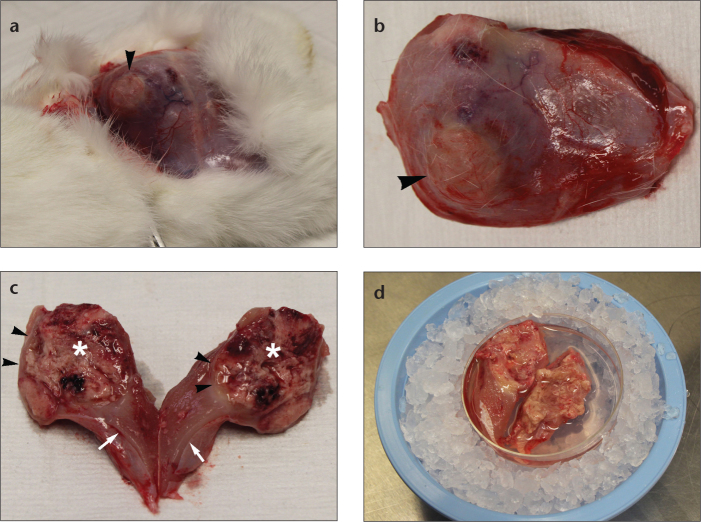
Figure 4. a–d.
Photograph (a) demonstrates gross appearance of exposed hind limb muscle containing tumor implant (arrowhead). Photograph (b) reveals explanted hind limb muscle containing tumor nodule (arrowhead). Photograph (c) shows bisected tumor explant demonstrating necrotic core (asterisk), peripheral viable tumor (arrowheads), and adherent muscle tissue (arrows). After removal of necrotic tumor and small viable tumor pieces for hepatic implantation, the remaining tumor is stored in RPMI medium and kept on ice for later processing as shown in photograph (d).
Liver tumor implantation via laparotomy
Intrahepatic implantation of solid tumor fragments is more reliable than the injection of a cell suspension. In a study by Virmani et al. (21), solid tumor implantation and injection of cell suspension were associated with success rates of 84% and 43%, respectively, as determined by evidence of tumor growth on magnetic resonance imaging and subsequent histopathological analysis. In a report by Chen et al. (22), implantation was successful in 95% of cases and injection in 35% of cases, and the latter was associated with a high rate of extrahepatic tumor growth. In an investigation by Sun et al. (23), open intrahepatic injection of tumor fragments achieved tumor growth in 96% of cases, compared with 64% of cases injected with tumor suspension. Of note, percutaneous image-guided tumor implantation techniques have also been reported (24).
For liver tumor implantation, the recipient rabbit is medicated for anesthetic induction with ketamine and dexmeditomidine, followed by intubation and maintenance with 1%–3% isoflurane delivery as needed to sustain a surgical plane of anesthesia. Meloxicam and one dose of enrofloxacin (5 mg/kg, subcutaneous) are provided before the procedure. After preparing and draping the abdomen in standard surgical fashion (Fig. 5), a vertically oriented subxyphoid mini-laparotomy incision is made with a 15-blade, and blunt dissection is used to expose the peritoneum through the avascular linea alba. Careful division of the peritoneum will permit visualization of the liver. Gentle manual or cotton tipped applicator traction may be used to expose the left lobe of the liver, which is drawn out from the incision (Fig. 5a). Care should be taken to avoid tearing of the liver capsule, and the exposed organ should be kept moist with wet gauze. At least one investigation suggests that the left lateral hepatic lobe is the preferred implantation site for VX2 tumors due to a more favorable angle of the feeding artery for later angiographic catheterization (25). Two vertical stab incisions are then made with the tip of an 11-blade, roughly 2–3 cm apart and 3–5 mm deep; hemostasis at the incision sites may be obtained with gentle manual compression. A single tumor fragment is then placed atop each incision and gently pushed into the parenchyma with the tip of a fine forceps (Fig. 5b). Care should be taken to avoid contact of the tumor fragment with other parts of the liver surface, other abdominal structures, or the abdominal wall in order to prevent nontarget implantation or metastases. The incisions are subsequently covered with a small piece of hemostatic surgical sponge, such as Surgicel (Ethicon, Somerville, New Jersey, USA) or Blood-STOP iX (PRN Pharmacal, Pensacola, Florida, USA), in order to limit bleeding (Fig. 5c). Judicious use of this material is recommended in order to lessen the potential for abdominal wall adhesion and intraperitoneal tumor dislodgment or ingrowth. After hemostasis is achieved, the abdominal incision may be closed in two layers using 3-0 PDS suture (Ethicon) for fascial repair and 5-0 Vicryl suture (Ethicon) for cutaneous apposition. Typically, the tumors will develop into discreet 2–3 cm nodules within 2–4 weeks of implantation (15) (Fig. 6). Serial liver ultrasonography follow-up, starting one week after tumor implantation, may be useful in documenting appropriate solid tumor growth prior to onset of internal necrosis (24); use of non-necrotic tumors will optimize evaluation of tumor response to locoregional therapy experiments.
Figure 5. a–c.
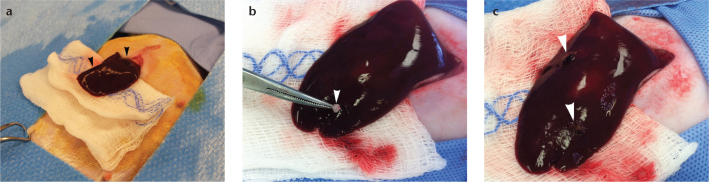
Photograph (a) demonstrates exposure of left medial hepatic lobe (arrowheads) through subxyphoid incision. Photograph (b) details forceps implantation of 1–2 mm3 tumor fragment (arrowhead). Photograph (c) shows stab incisions covered with hemostatic surgical sponge (arrowheads).
Figure 6. a, b.
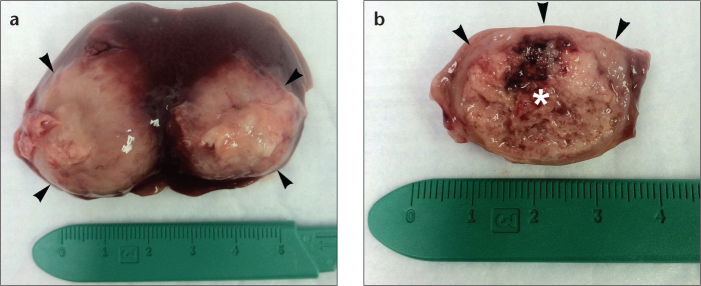
Photograph (a) of resected liver specimen demonstrates gross appearance of 2–3 cm hepatic tumors (arrowheads) after four weeks of growth. Photograph (b) of transected tumor nodule shows necrotic core (asterisk) and peripheral rim of viable tumor (arrowheads).
Of note, the method of surgical tumor implantation into rabbit livers minimizes the potential of metastasis. Nonetheless, signs of metastasis to the lungs include increased respiratory rate and effort. Abdominal breathing may be present along with nasal flaring. Metastasis to the brain would cause lethargy, decreased alertness, and/or behavioral changes (e.g., unusual aggressiveness).
VX2 tumor suspension preparation
Preparation of VX2 inoculum from freshly harvested hind limb tumors requires a cell strainer, a conical polypropylene tube, syringes, and RPMI media (Fig. 7a). Viable tumor is scraped from explanted tumor specimens using a 15-blade. The tumor scrapings are collected and strained using a 40-micron cell strainer (BD Biosciences, Franklin Lake, New Jersey, USA) into a conical polypropylene 50 mL tube (BD Biosciences) (Fig. 7b). A single cell suspension is made using RPMI media to wash cells through the strainer. The resulting mixture is centrifuged at 1600 rotations per minute for eight minutes (Fig. 7c), after which the supernatant liquid is discarded and the precipitant cellblock is mixed with methylcellulose medium in a 1:1 volumetric ratio. The fresh VX2 suspension is injected into the hind limbs of a new donor rabbit for tumor line maintenance. Excess VX2 cellblock samples may be saved by storing in cryogenic vials (CryoTube vials; Sigma-Aldrich) and freezing in liquid nitrogen. To this end, approximately 1 mL VX2 cell aliquots are dispensed into 1.8 mL cryogenic vials, which are sealed with paraffin film and directly submerged into a liquid nitrogen storage tank.
Figure 7. a–c.

Photograph (a) demonstrates scraped tumor tissue passed through 40-micron cell strainer (black arrowhead) using 5 mL syringe plunger (black arrow) and RPMI wash. Care is taken to maintain ice cooling of the mixture. Photograph (b) depicts strained tumor suspension prior to centrifugation. Photograph (c) exhibits VX2 precipitate (arrowheads) and supernatant liquid (arrows) following centrifugation.
Angiographic utilization of VX2 liver tumor
Implanted VX2 tumors may be used to investigate transarterial therapies. For arteriography, the liver tumor laden rabbit is medicated and maintained under inhaled anesthesia in a manner identical to that for liver tumor implantation. After preparing and draping the groin area in standard fashion, the femoral artery is accessed through a surgical cut-down; the vessel is generally too small for percutaneous ultrasound-guided access. A 2–3 cm long groin incision is made using a 15-blade, with blunt dissection down to the femoral bundle. The femoral artery is gently teased apart from the accompanying nerve and vein, and isolated atop an instrument handle for support (Fig. 8). Vascular access may then be achieved using standard Seldinger technique and a 21-gauge needle and 0.018-inch guidewire, with subsequent placement of a 3 F vascular sheath (Cook Medical, Bloomington, Indiana, USA). Using fluoroscopic guidance with a C-arm unit, a 2 F catheter (JB1, Cook Medical) is advanced over a guidewire; the celiac, common hepatic, and proper hepatic arteries are selectively catheterized; and arteriography is performed via injections of iohexol (Omnipaque-300; Amersham Health, Princeton, New Jersey, USA) (Fig. 9). Care is taken during catheter and guidewire manipulations to avoid vascular spasm, which occurs easily but may be treated with nitroglycerine. After catheter placement is confirmed in the vascular distribution of the VX2 tumors, the desired therapeutic agent may be administered through the catheter. After completion of the infusion, the catheter is removed and the femoral artery is ligated using 3-0 silk suture (Ethicon) to obtain hemostasis, following which collateral vessels will preserve blood flow to the lower extremities. The incision may be closed in a single layer using 5-0 Vicryl suture (Ethicon), and the animal is aroused and recovered from anesthesia, and followed-up clinically until its time of sacrifice.
Figure 8. a–c.
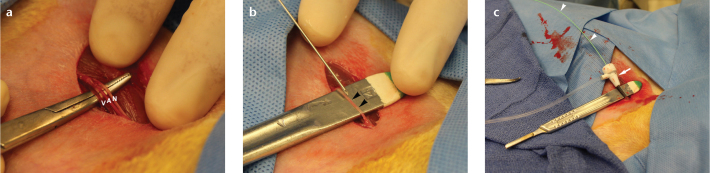
Vascular access. Photograph (a) demonstrates exposure of femoral nerve (N), artery (A), and vein (V). Photograph (b) depicts needle cannulation of isolated femoral artery (arrowheads) using instrument handle for support. Photograph (c) displays femoral arterial catheterization using 3 F sheath (arrow) and 2 F catheter (arrowheads).
Figure 9. a–c.
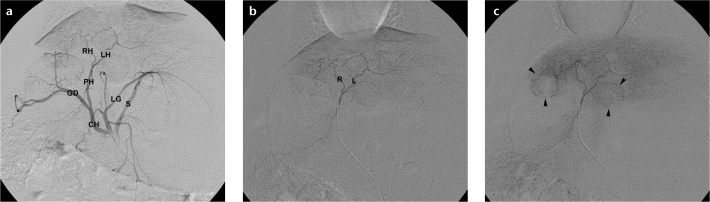
Digital subtraction arteriograms demonstrate rabbit liver vasculature. Celiac arteriogram (a) shows splenic (S), left gastric (LG), common hepatic (CH), gastroduodenal (GD), proper hepatic (PH), left hepatic (LH), and right hepatic (RH) arteries. Proper hepatic arteriogram (b) better delineates left (L) and right (R) hepatic arteries. Delayed proper hepatic arteriogram (c) displays two hypervascular VX2 liver tumors (arrowheads).
Conclusion
The rabbit VX2 tumor model is a useful tool for the study of hepatocellular carcinoma and other neoplasms, and successive application of the methods described herein over 2–3 week intervals will allow investigators to sustain an active VX2 tumor lab operation. Although the high rate of spontaneous tumor necrosis may limit therapeutic efficacy studies, modified VX2 lines exhibiting greater viability have been developed (26). While a description of angiographic utilization was provided, liver tumors may also be used for other interventions, such as percutaneous thermal ablation (27) and irreversible electroporation (28). Regardless of the interventional procedure investigated, a working knowledge of the technical methods necessary for utilization of the VX2 tumor will benefit interventional radiology investigators desiring pursuit of translational research with this model.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Rous P, Beard JW. The progression to carcinoma of virus-induced rabbit papillomas (Shope) J Exp Med. 1935;62:523–548. doi: 10.1084/jem.62.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kidd JG, Rous P. A transplantable rabbit carcinoma originating in a virus-induced papilloma and containing the virus in masked or altered form. J Exp Med. 1940;71:813–838. doi: 10.1084/jem.71.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galasko CS, Muckle DS. Intrasarcolemmal proliferation of the VX2 carcinoma. Br J Cancer. 1974;29:59–65. doi: 10.1038/bjc.1974.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maruyama H, Matsutani S, Saisho H, et al. Sonographic shift of hypervascular liver tumor on blood pool harmonic images with definity: time-related changes of contrast-enhanced appearance in rabbit VX2 tumor under extra-low acoustic power. Eur J Radiol. 2005;56:60–65. doi: 10.1016/j.ejrad.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 5.van Es RJ, Dullens HF, van der Bilt A, Koole R, Slootweg PJ. Evaluation of the VX2 rabbit auricle carcinoma as a model for head and neck cancer in humans. J Craniomaxillofac Surg. 2000;28:300–307. doi: 10.1054/jcms.2000.0165. [DOI] [PubMed] [Google Scholar]
- 6.Lee JM, Kim SW, Chung GH, Lee SY, Han YM, Kim CS. Open radio-frequency thermal ablation of renal VX2 tumors in a rabbit model using a cooled-tip electrode: feasibility, safety, and effectiveness. Eur Radiol. 2003;13:1324–1332. doi: 10.1007/s00330-002-1658-x. [DOI] [PubMed] [Google Scholar]
- 7.Frank JA, Girton M, Dwyer AJ, et al. A reproducible model of metastatic brain and ocular tumor by hematogenous inoculation of the VX2 tumor in rabbits. J Neurosurg. 1987;67:106–109. doi: 10.3171/jns.1987.67.1.0106. [DOI] [PubMed] [Google Scholar]
- 8.Anayama T, Nakajima T, Dunne M, et al. A novel minimally invasive technique to create a rabbit VX2 lung tumor model for nano-sized image contrast and interventional studies. PLoS One. 8:e67355. doi: 10.1371/journal.pone.0067355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang WH, Liebert M, Price RE, Cromeens DM, Lin JS, Grossman HB. Extravesical cryosurgical approach for VX2 bladder tumor in rabbits. Urol Res. 2001;29:345–349. doi: 10.1007/s002400100204. [DOI] [PubMed] [Google Scholar]
- 10.Rhee TK, Ryu RK, Bangash AK, et al. Rabbit VX2 tumors as an animal model of uterine fibroids and for uterine artery embolization. J Vasc Interv Radiol. 2007;18:411–418. doi: 10.1016/j.jvir.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Burgener FA. Peripheral hepatic artery embolization in rabbits with VX2 carcinomas of the liver. Cancer. 1980;46:56–63. doi: 10.1002/1097-0142(19800701)46:1<56::aid-cncr2820460113>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 12.Hoye RC, Thomas LB, Riggle GC, Ketcham A. Effects of neodymium laser on normal liver and Vx2 carcinoma transplanted into the liver of experimental animals. J Natl Cancer Inst. 1968;41:1071–1082. [PubMed] [Google Scholar]
- 13.Handal JA, Schulz JF, Florez GB, Kwok SC, Khurana JS, Samuel SP. Creation of rabbit bone and soft tissue tumor using cultured VX2 cells. J Surg Res. 179:e127–132. doi: 10.1016/j.jss.2012.02.061. [DOI] [PubMed] [Google Scholar]
- 14.Eifler AC, Lewandowski RJ, Virmani S, et al. Development of a VX2 pancreatic cancer model in rabbits: a pilot study. J Vasc Interv Radiol. 2009;20:1075–1082. doi: 10.1016/j.jvir.2009.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang D, Bangash AK, Rhee TK, et al. Liver tumors: monitoring embolization in rabbits with VX2 tumors--transcatheter intraarterial first-pass perfusion MR imaging. Radiology. 2007;245:130–139. doi: 10.1148/radiol.2451061689. [DOI] [PubMed] [Google Scholar]
- 16.Ma HL, Xu YF, Qi XR, Maitani Y, Nagai T. Superparamagnetic iron oxide nanoparticles stabilized by alginate: pharmacokinetics, tissue distribution, and applications in detecting liver cancers. Int J Pharm. 2008;354:217–226. doi: 10.1016/j.ijpharm.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 17.Choi YH, Chung JW, Son KR, et al. Novel intraarterial therapy for liver cancer using ethylbromopyruvate dissolved in an iodized oil. Acad Radiol. 2011;18:471–478. doi: 10.1016/j.acra.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Gaba RC, Baumgarten S, Omene BO, et al. Ethiodized oil uptake does not predict doxorubicin drug delivery after chemoembolization in VX2 liver tumors. J Vasc Interv Radiol. 2012;23:265–273. doi: 10.1016/j.jvir.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 19.Xia X, Li X, Feng G, Zheng C, Liang H, Zhou G. Intra-arterial interleukin-12 gene delivery combined with chemoembolization: anti-tumor effect in a rabbit hepatocellular carcinoma (HCC) model. Acta Radiol. 2013;54:684–689. doi: 10.1177/0284185113480072. [DOI] [PubMed] [Google Scholar]
- 20.National Cancer Institute, Division of Cancer Treatment Diagnosis and Treatment Tumor/Cell Line Repository. Available at: http://dctd.cancer.gov/Program-Pages/dtp/tools_tumor_cellline.htm. Accessed September 28, 2013.
- 21.Virmani S, Harris KR, Szolc-Kowalska B, et al. Comparison of two different methods for inoculating VX2 tumors in rabbit livers and hind limbs. J Vasc Interv Radiol. 2008;19:931–936. doi: 10.1016/j.jvir.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen JH, Lin YC, Huang YS, Chen TJ, Lin WY, Han KW. Induction of VX2 carcinoma in rabbit liver: comparison of two inoculation methods. Lab Anim. 2004;38:79–84. doi: 10.1258/00236770460734434. [DOI] [PubMed] [Google Scholar]
- 23.Sun JH, Zhang YL, Nie CH, et al. Considerations for two inoculation methods of rabbit hepatic tumors: Pathology and image features. Exp Ther Med. 2012;3:386–390. doi: 10.3892/etm.2011.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo W, Zhou X, Zheng X, et al. Role of sonography for implantation and sequential evaluation of a VX2 rabbit liver tumor model. J Ultrasound Med. 2010;29:51–60. doi: 10.7863/jum.2010.29.1.51. [DOI] [PubMed] [Google Scholar]
- 25.Lee KH, Liapi E, Buijs M, et al. Considerations for implantation site of VX2 carcinoma into rabbit liver. J Vasc Interv Radiol. 2009;20:113–117. doi: 10.1016/j.jvir.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pascale F, Ghegediban SH, Bonneau M, et al. Modified model of VX2 tumor overexpressing vascular endothelial growth factor. J Vasc Interv Radiol. 2012;23:809–817. doi: 10.1016/j.jvir.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Hansler J, Neureiter D, Wasserburger M, et al. Percutaneous US-guided radiofrequency ablation with perfused needle applicators: improved survival with the VX2 tumor model in rabbits. Radiology. 2004;230:169–174. doi: 10.1148/radiol.2301021136. [DOI] [PubMed] [Google Scholar]
- 28.Lee EW, Wong D, Tafti BA, et al. Irreversible electroporation in eradication of rabbit VX2 liver tumor. J Vasc Interv Radiol. 2012;23:833–840. doi: 10.1016/j.jvir.2012.02.017. [DOI] [PubMed] [Google Scholar]