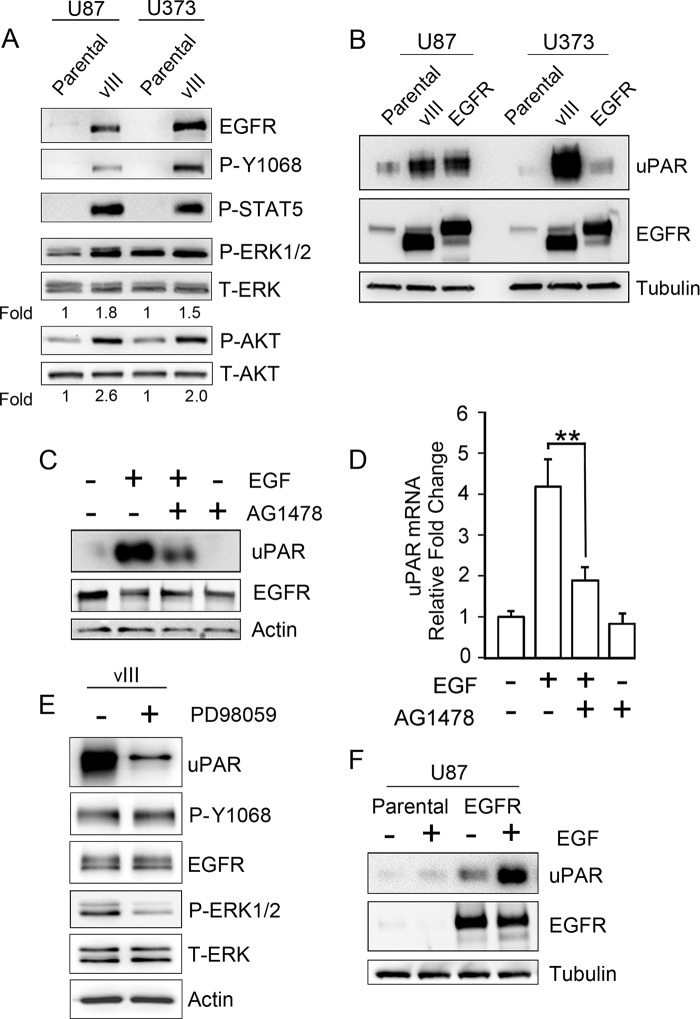
FIGURE 1.
EGFR signaling increases expression of cellular uPAR in human GBM cells. A, immunoblot analysis comparing EGFR expression in U87MG and U373MG parental cells and in the same cells that express EGFRvIII. Activation of the EGFR (P-Y1068), ERK1/2, Akt, and STAT5 was assessed after serum-starving cells for 18 h. Mean relative signal intensities for P-ERK1/2 and P-Akt relative to T-ERK1/2 and T-Akt are shown below the blots (n = 3). B, immunoblot analysis comparing cellular uPAR in parental U87MG and U373MG cells and in the same cells that overexpress WT-EGFR or express EGFRvIII (vIII). uPAR immunoblots were performed using the identical samples but separate blots because the antibody requires nonreducing conditions. Blots were re-probed for tubulin as a control for load. C, U373MG cells that were serum-starved for 18 h were pretreated with 50 nm AG1478 for 2 h, as indicated (+), and then with 10 ng/ml EGF for 6 h, as indicated (+). Immunoblot analysis was performed to detect cellular uPAR, EGFR, and actin as a control for load. D, qPCR was performed to detect uPAR mRNA after completing the identical incubations as in C (mean ± S.D. relative to control, n = 3; **, p < 0.01). E, EGFRvIII-expressing U373MG cells were serum-starved for 18 h and subsequently treated (+) or untreated (−) for 18 h with 25 μm PD98059 in serum-free medium. Immunoblotting was performed to assess the indicated antigens. F, parental and WT-EGFR-expressing U87MG cells were treated with 10 ng/ml EGF (+) or with vehicle (−) in serum-free medium for 18 h. Immunoblot analysis was then performed.