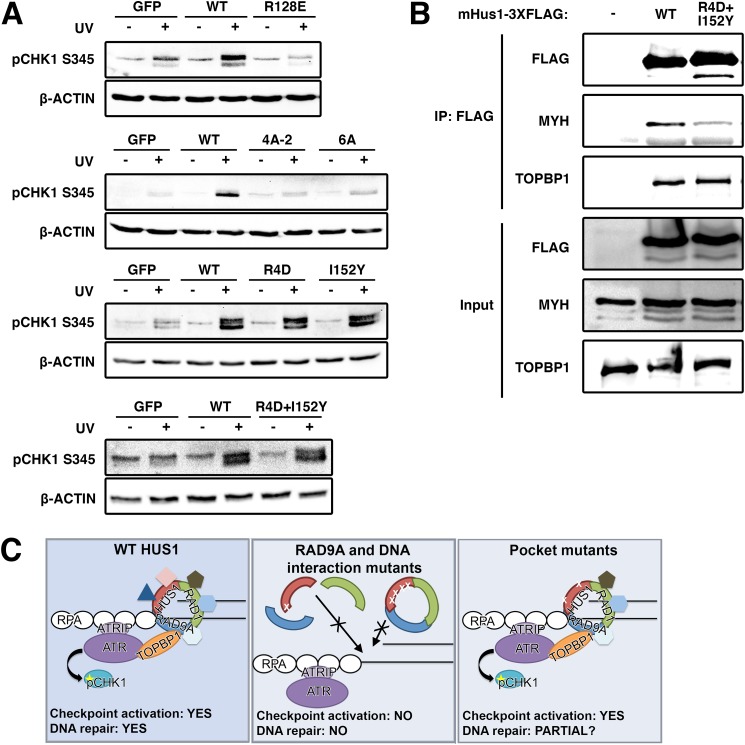
FIGURE 7.
9-1-1-dependent checkpoint signaling requires clamp formation and DNA associations but not HUS1 outer surface pocket function, which is necessary for effector interactions. A, DNA damage-induced CHK1 phosphorylation is hampered in HUS1 clamp formation and DNA interaction mutants but is intact for HUS1 outer surface pocket mutants. Lysates from cells treated with 0 or 100 J/m2 UV were immunoblotted using antibodies specific for phospho-CHK1 or β-actin. B, HUS1 pocket mutant R4D,I152Y is impaired for interaction with base excision repair protein MYH. Lysates prepared from HEK293T cells overexpressing 3XFLAG-tagged WT or R4D,I152Y mHUS1 proteins were immunoprecipitated with antibody specific for FLAG and immunoblotted using antibodies specific for MYH and TOPBP1. C, model for HUS1-mediated function in DNA damage response. WT HUS1 forms 9-1-1 clamps, localizes to DNA damage sites, and mediates ATR checkpoint signaling and DNA repair functions. When the RAD9A-interacting residue is dysfunctional, HUS1 cannot form 9-1-1 clamp, causing loss of all downstream functions. HUS1 mutants defective for DNA interactions are still able to form 9-1-1 clamps but cannot localize to DNA damage sites, similarly causing loss of all downstream functions. Only HUS1 pocket mutants are able to form 9-1-1 clamps, localize to DNA lesions, and activate ATR for checkpoint signaling. However, checkpoint-independent functions of HUS1 are perturbed in the pocket mutants, probably causing increased genotoxin hypersensitivity due to impaired DNA repair.