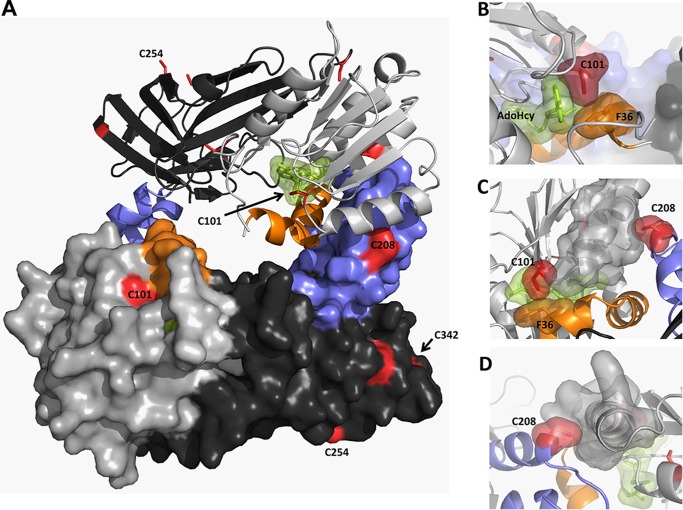
FIGURE 7.
Cysteine residues in rPRMT1. Shown is the PRMT1 dimer (PDB code 1OR8) colored as described in the text (AdoMet binding domain in light gray, β barrel domain in dark gray, dimerization arm in blue, αγ-loop-αZ in orange, AdoHcy in green, and cysteine residues in red). Residues 26–39 were modeled on the basis of the position of this helix in the PRMT3 structure (PDB code 1F3L). A, PRMT1 dimer. B, surface representation showing close active site interactions between AdoHcy, Cys101, and Phe36. C, top view of the dimerization arm in one monomer interacting with the AdoMet binding site in the other monomer. D, back view showing the packing of Cys208 in one monomer with the α helix in the AdoMet binding domain of the other monomer.