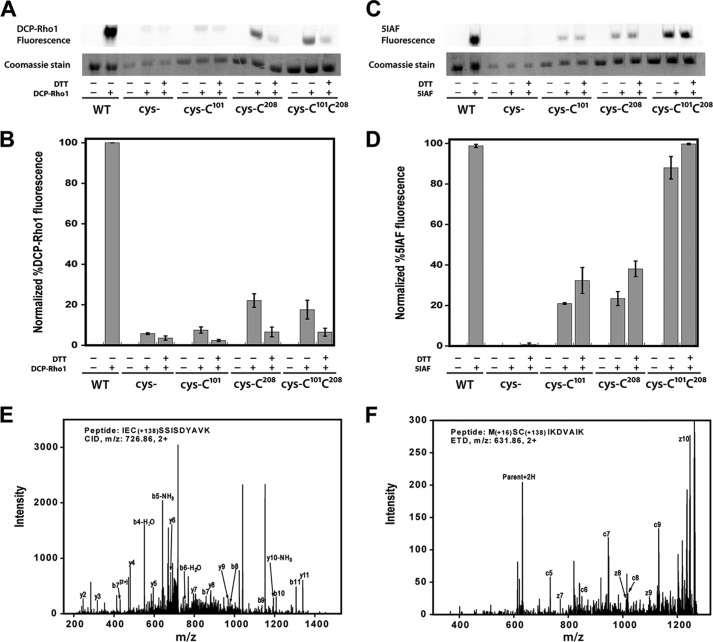
FIGURE 8.
Sulfenic acid detection and free thiol content in PRMT1. A–D, air-oxidized wild-type PRMT1 (WT), cys-, cys-Cys101, cys-Cys208, and cys-Cys101Cys208 were denatured in 6 m urea and incubated with 1 mm DTT or buffer prior to addition of 10 μm DCP-Rho1 or 2.5 mm 5IAF. Labeled samples were resolved by SDS-PAGE. A, representative image of the rhodamine fluorescence signal and the corresponding Coomassie bands. B, graphical representation of triplicate gel analysis. Normalized percent DCP-Rho1 fluorescence represents the percentage of fluorescent signal divided by the amount of protein observed in the Coomassie bands, which is interpreted as the relative amount of sulfenic acid present. C, representative image of the 5IAF fluorescence signal and the corresponding Coomassie bands. D, graphical representation of triplicate 5IAF gel analysis, interpreted as relative amount of free thiols present. E and F, representative MS/MS fragmentation spectra for peptides containing dimedone-modified sulfenic acids in PRMT1. E, the CID fragments of peptide IECSSISDYAVK labeled with dimedone at Cys101. F, ETD fragments of peptide MCSIKDVAIK labeled with dimedone at Cys208. The mass shift by the modification is 138.068 (exact number), as denoted in the peptide sequence.