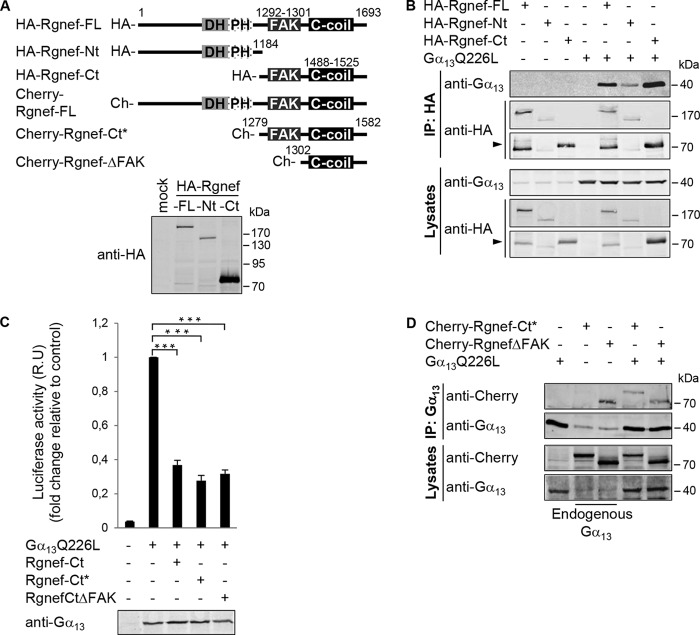
FIGURE 5.
Rgnef C-terminal domain association with activated Gα13Q226L. A, schematic of full-length and truncated Rgnef constructs as HA- or mCherry- fusion proteins. Lower panel, expression of HA-tagged full-length, Nt(1–1184), and Ct(1185–1693) Rgnef constructs in HEK293 cells by anti-HA immunoblotting. B, HEK293 cells were transfected with HA-tagged vectors encoding for Rgnef full-length, Rgnef-Nt(1–1184), Rgnef-Ct(1185–1693), and/or Gα13Q226L. Cell lysates were immunoprecipitated as described before. Data are representative of at least three independent experiments. Arrow points to Rgned-Ct. C, Rgnef-Ct domain constructs inhibit Gα13Q226L-mediated SRF activation. HEK293 cells were transfected with pSRE.L and pRL-TK, together with either empty vector, vector encoding Gα13QL, and/or Rgnef -Ct(1185–1693), Rgnef-Ct*(1279–1582), and Rgnef ΔFAK(1302–1582). After 24 h of transfection, cells were serum-starved overnight, and then SRF activities of cell lysates were measured using the Dual-Luciferase assay kit (Promega). Anti-Gα13 immunoblotting shows equal expression in cell lysates. Data are means ± S.E. of four independent experiments, each conducted in triplicate (*, p < 0.05; **, p < 0.005; two-tailed t test). D, HEK293 cells expressing Gα13 in presence or absence of mCherry-Rgnef-FL, mCherry-Rgnef-Ct*, and mCherry-RgnefΔFAK were immunoprecipitated using anti-Gα13 antibody and subjected to Western blot analysis. Cells lysates were analyzed in parallel. Full-length Rgnef, Rgnef-Ct, and ΔFAK were detected by anti-Rgnef, and Gα13 was detected using anti-Gα13 immunoblotting. Data are representative of four independent experiments.