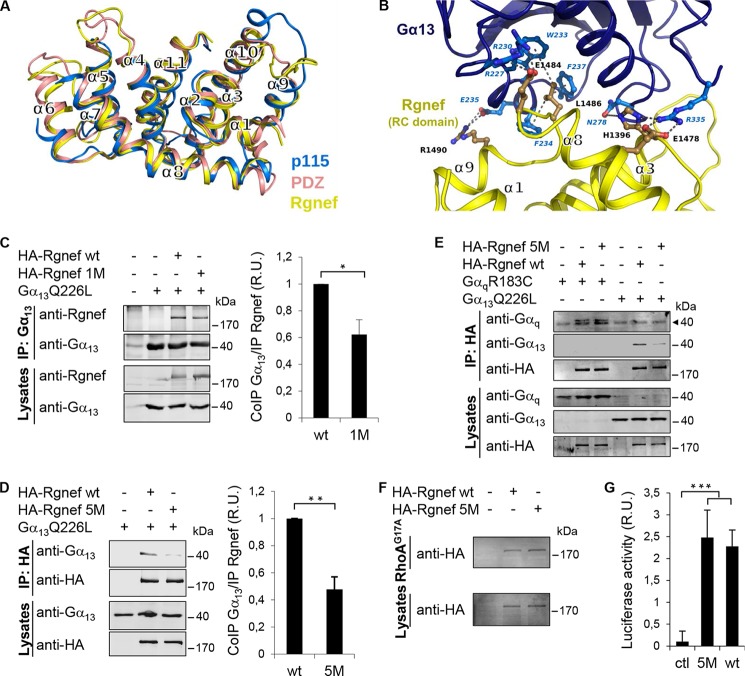
FIGURE 7.
Models of putative Rgnef RH-like domain and the complex with Gα13. A, structural superposition of the RH domains of human PDZ-RhoGEF (Protein Data Bank code 3CX8; salmon), p115 RhoGEF (Protein Data Bank code 3AB3; blue) and our computer model of the domain of Rgnef (yellow). B, computer model of the domain of Rgnef (yellow) in complex with Gα13 (blue). Putative residues of charged or hydrophobic side chain interactions are shown in blue. Residues mutated in the Rgnef-5 M are shown in black (corresponding to human His-1396, Glu-1478, Glu-1484, Leu-1486, and Arg-1490). C and D, HEK293 cells were transfected with vectors encoding for HA-Rgnef (full length), HA-Rgnef 1M (C), or HA-Rgnef 5M (D) with or without Gα13Q226L. HA-tagged Rgnef constructs were co-immunoprecipitated with antibodies to Gα13 and detected by Li-COR immunoblotting. Graphical results show immunoblotting band intensities from three independent experiments expressed as fold induction with respect to immunoprecipitated Rgnef wild type and normalized by the total expression level of Rgnef. Values are means ± S.D. (*, p < 0.05; **, p < 0.005; two-tailed t test). E, HEK293 cells expressing HA-Rgnef or HA-Rgnef5M with or without GαqR183C or Gα13Q226L were co-immunoprecipitated with HA tag antibodies and detected by Li-COR immunoblotting. The arrowhead points to an unspecific band detected with the anti-Gq monoclonal antibody. F, HEK293 cells expressing HA-Rgnef or HA-Rgnef 5M were incubated with RhoAG17A-GST beads and visualized by anti-HA tag immunoblotting. G, HEK293 cells were transfected with pSRE.L and pRL-TK, together with either empty vector (Ctl), vector encoding Rgnef or Rgnef5M and SRF activities were measured. Data are means ± S.E. of three independent experiments, each conducted in duplicate (***, p < 0.001).